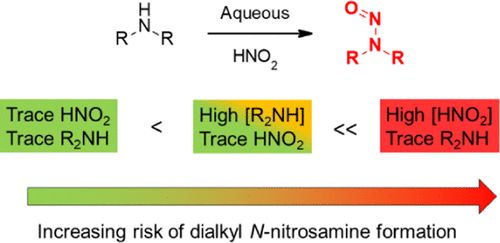
@Yosukemino We are commonly asked ‘what should be the Nitrate spec for Excipient/API/Ingredient?’, Nitrate on its own is not the sole cause of Nitrosamien formation. Only risk assessment and examination of the full process allows to make that call and define what levels if any represent a risk.
@DAB had a post in reference to the APIC guide, so I’m interested if she can share her experience based on the situation that you describe. It’s a reality that some (not all) API/Excipient manufacturers limit their assessment to a disclaimer on their CoA regarding the risk or content of nitrate/secondary amine. What’s the backup plan for this scenario.
Although not the same, but this publication shines some light on the risk of Nitrosamine formation using the content of Nitrate is water as a model. https://doi.org/10.1021/acs.oprd.0c00224
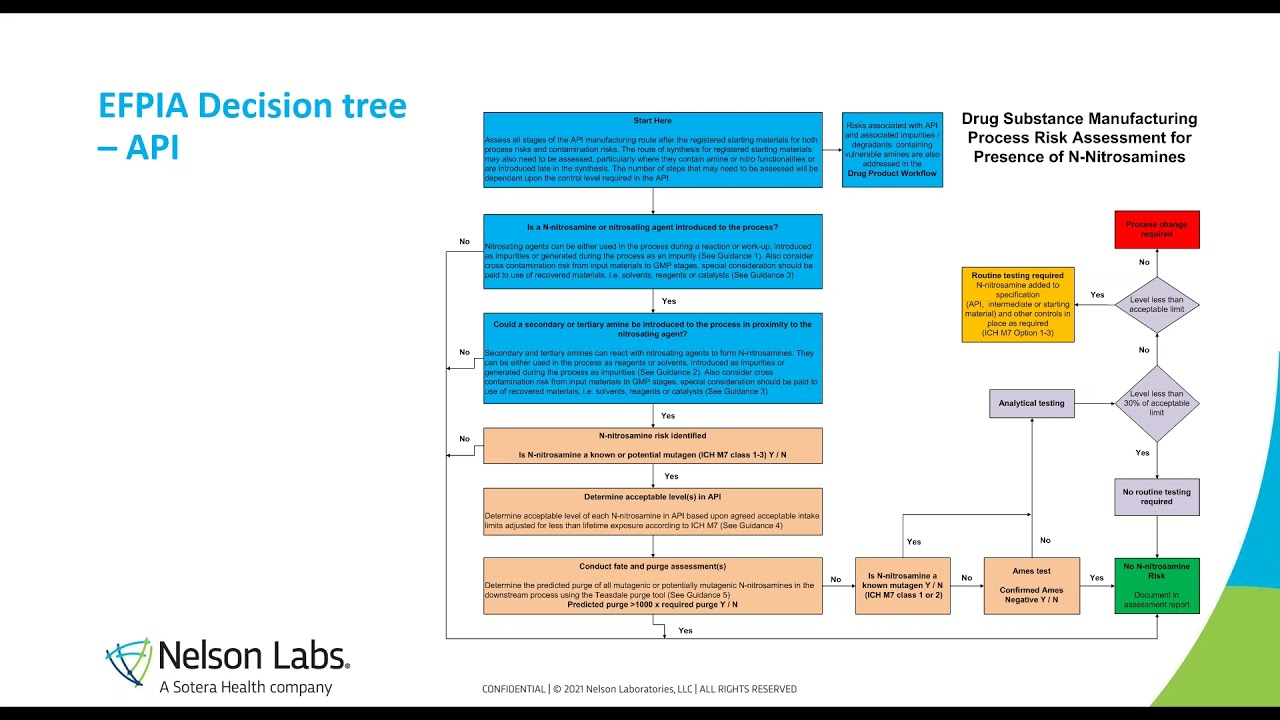
Few months ago @AndyTeasdale and Ank Reumer (Nelson Labs) delivered a webinar ‘Hunt for N-Nitrosamines in Medicinal Products’ that illustrate some of these concepts in real scenarios