Dear @sushantkamath,
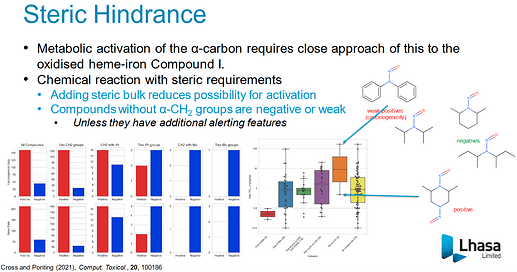
Thank you for asking good questions. You asked why is 0,3 = 2; 1,3 = 3; 2,2=1 and 2,3 =1. And I’m not sure of the answer, but I know compounds with less score are vulnerable to metabolic activation by CYP. The alfa-hydroxylation step is the key to nitrosamines’ carcinogenicity. I add the slide and link to Ponting’s presentation.
I guess the score was determined from the results of TD50 of lots of nitrosamines. In other words, the scoring system was built through validation with nitrosamines’ toxicological data. Compounds with low scores have a tendency to be potent in carcinogenicity. And visualization thread may be helpful to understand the scoring system.
If you need further help, do not hesitate to ask us.