As we discussed, “dipines” are considered impossible to be synthesized. Example 1 may not be appropriate.
Example 1 –N-Nitroso-felodipine
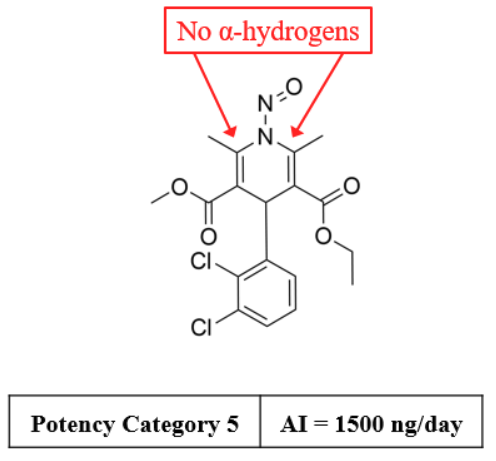
As we discussed, “dipines” are considered impossible to be synthesized. Example 1 may not be appropriate.
Example 1 –N-Nitroso-felodipine
