For the specific question - my interpretation is tht the (0,2) combination is worth a CPCA score of 2, before the activating/deactivating features, if the side chain is specifically ethyl, which puts ethyl groups in the same bucket as the (0,3) methyl group. This is based on the comparable potency and metabolic lability of ethyl and methyl groups. All other CH2 groups are less labile and are worth a CPCA score of 3.
Indeed, the increased steric bulk infers a dramatic reduction in potency. We essentially have three cases here in appendix A table 2, with some modulation between methyl and larger groups.
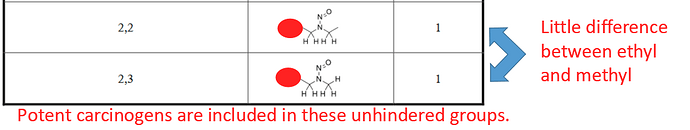
If the most-hindered side is CH2, then whether the right-hand side is CH2 or CH3 makes little difference, both sides can react and highly-reactive diazonium ions can form, hence the score of 1 - reflected in a number of potent carcinogens that match this unhindered pattern.
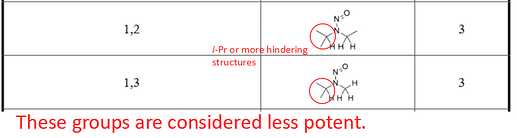
Isopropyl groups, as I have been saying for a number of years, lead to a dramatic reduction in potency, hence the score of 3 for either (1,3) or (1,2).
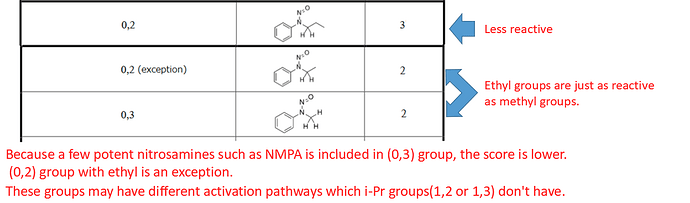
Aryl groups are interesting, because there are a few potent examples such as nitrosomethyl aniline (NMA or NMPA) which explains the lower score for (0,3), and ethyl groups are just as reactive as methyl hence the exception in (0,2). All other groups (other than activating groups) are less labile, making the rest of (0,2) have a score of 3. These also have some potential alternative mechanisms not available to the isopropyl ones, hence the lower scores for NMA and nitrosoethylaniline and ring-substituted derivatives.
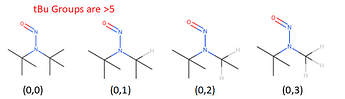
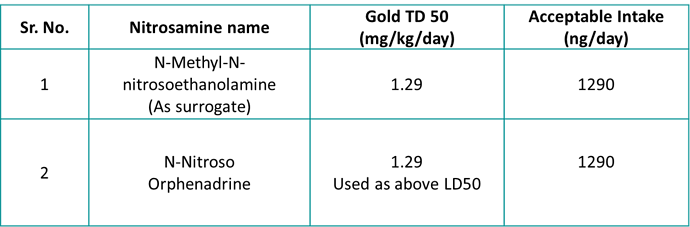
As a caveat, tert-butyl groups [(0,0) (0,1), (0,2), (0,3) where the 0 is sp3], as well as the fully-hindered (0,1) and (1,1) have an effective CPCA score of >5, enough to make them category 4 if they did need the CPCA but of course don’t need it, and the only (3,3) is NDMA - which of course has a compound-specific limit. This is why not all permutations are in the CPCA.
(I really should have put my surname in my username here… I am the Ponting you mention)
A morning spent feverishly calculating Potency Category values for various molecules - cross checking some against newly published limits (making sure I was doing it correctly and agreeing with the EMA) and creating new limits!!
In many cases better than I had dared to hope for!
Finally, as has been said all along, targets to work to. Now, as an industry, we can look at:-
- are our methods good enough to detect at the new limits (and down to 10% of them)?
- are levels in products acceptable to these new limits?
- how to reduce levels in products that are still problematic.
Fantastic to see such collaboration between the industry as whole to drive us to this position, being led by the science, with some outstanding contributions from individuals and organisations.
Now if we can only harmonize these limits globally (over to you FDA, ANVISA etc.) and also harmonize Less Than Lifetime limits. Or am I just greedy?
Indeed a privilege to hear from you Dr. Ponting. Thank you for the detailed explanation. I want to congratulate you for the immense contribution you have made in this field. We were all waiting for agencies to accept a chemistry approach to set AI limits instead of relying on only in vivo studies. This new guideline comes as a breather to the pharmaceutical industry.
Thank You
Regards
Sushant
Mark can you share your preliminary assessment on structures? I would be a tremendous didactic contribution!
Community Members !!! @trust_user_a @trust_user_c @Nitrosamines_Analyzer
By now, you are probably aware of the significant update published last Friday (July 7th) on EMA’s 𝐐𝐮𝐞𝐬𝐭𝐢𝐨𝐧𝐬 𝐚𝐧𝐝 𝐚𝐧𝐬𝐰𝐞𝐫𝐬 𝐟𝐨𝐫 𝐦𝐚𝐫𝐤𝐞𝐭𝐢𝐧𝐠 𝐚𝐮𝐭𝐡𝐨𝐫𝐢𝐬𝐚𝐭𝐢𝐨𝐧 𝐡𝐨𝐥𝐝𝐞𝐫𝐬/𝐚𝐩𝐩𝐥𝐢𝐜𝐚𝐧𝐭𝐬 𝐨𝐧 𝐭𝐡𝐞 𝐂𝐇𝐌𝐏 𝐎𝐩𝐢𝐧𝐢𝐨𝐧 𝐟𝐨𝐫 𝐭𝐡𝐞 𝐀𝐫𝐭𝐢𝐜𝐥𝐞 𝟓(𝟑) 𝐨𝐟 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐢𝐨𝐧 (𝐄𝐂) 𝐍𝐨 𝟕𝟐𝟔/𝟐𝟎𝟎𝟒 𝐫𝐞𝐟𝐞𝐫𝐫𝐚𝐥 𝐨𝐧 𝐧𝐢𝐭𝐫𝐨𝐬𝐚𝐦𝐢𝐧𝐞 𝐢𝐦𝐩𝐮𝐫𝐢𝐭𝐢𝐞𝐬 𝐢𝐧 𝐡𝐮𝐦𝐚𝐧 𝐦𝐞𝐝𝐢𝐜𝐢𝐧𝐚𝐥 𝐩𝐫𝐨𝐝𝐮𝐜𝐭𝐬 - 𝙒𝙝𝙞𝙘𝙝 𝙡𝙞𝙢𝙞𝙩𝙨 𝙖𝙥𝙥𝙡𝙮 𝙛𝙤𝙧 𝙣𝙞𝙩𝙧𝙤𝙨𝙖𝙢𝙞𝙣𝙚𝙨 𝙞𝙣 𝙢𝙚𝙙𝙞𝙘𝙞𝙣𝙖𝙡 𝙥𝙧𝙤𝙙𝙪𝙘𝙩𝙨?
But what you did not know is about the insightful conversation we are hosting here in the Nitrosamine Exchange about the update. Even graphics examples have been generated to illustrate the guidance’s ranking framework!
Kudos to our community members @Yosukemino @Pradpharma @sushantkamath @David and @lucas10mauriz for driving the whole discussion about the guidance over the weekend as soon as the update got published!
This week, the industry is meeting EMA’s Nitrosamine Implementation oversight group (NIOG) to discuss the guidance, and I’m sure additional details will also emerge from this meeting.
Stay tuned and share any insight!
Of course @Naiffer_Host.
Back of an envelope scribblings at the moment, but will pull together something a little more explanatory tomorrow and share.
Thanks for sharing! This post and all of the conversation that has been shared by others is very helpful to inform others of these very important changes.
It is worth noting that while this is great progress, the CPCA categories are still quite conservative (which is acknowledged by EMA) and read-across might provide a more appropriate basis for an AI. For example, EMA has an AI of 78,000 ng/d for nitroso-mefenamic acid based on read-across from nitroso-diphenylamine. If they used a CPCA-derived AI rather than read-across, the limit would be 1500 ng/day (52-fold lower). It’s important to continue to have the flexibility to use read-across when it can be justified, so hopefully a CPCA-derived AI is treated as a refined default (analogous to a tiered TTC approach) and not a firmly established AI.
Dear @David
Thank you for your helpful and informative explanation. Of course, I remember you helped us in this community many times. And I want to illustrate your explanation to understand nitrosamines deeply.
If the most-hindered side is CH2, then whether the right-hand side is CH2 or CH3 makes little difference, both sides can react and highly-reactive diazonium ions can form, hence the score of 1 - reflected in a number of potent carcinogens that match this unhindered pattern.
Isopropyl groups, as I have been saying for a number of years, lead to a dramatic reduction in potency, hence the score of 3 for either (1,3) or (1,2).
Aryl groups are interesting, because there are a few potent examples such as nitrosomethyl aniline (NMA or NMPA) which explains the lower score for (0,3), and ethyl groups are just as reactive as methyl hence the exception in (0,2). All other groups (other than activating groups) are less labile, making the rest of (0,2) have a score of 3. These also have some potential alternative mechanisms not available to the isopropyl ones, hence the lower scores for NMA and nitrosoethylaniline and ring-substituted derivatives.
As a caveat, tert-butyl groups [(0,0) (0,1), (0,2), (0,3) where the 0 is sp3], as well as the fully-hindered (0,1) and (1,1) have an effective CPCA score of >5, enough to make them category 4 if they did need the CPCA but of course don’t need it, and the only (3,3) is NDMA - which of course has a compound-specific limit. This is why not all permutations are in the CPCA.
If I made a mistake, please point it out.
N-Nitroso Orphenadrine.
Nitroso Orphenadrine AI limit 18ng/day currently published EMA Q&A Rev.16 aapendix1 as per option 1.
Although can we justify the limit based on read-across approach?
Toxicology data information for one chemical N-Methyl-N-nitrosoethanolamine (the surrogate chemical) is used to predict the same for another chemical (the target chemical), which is considered to be “similar” on the basis of structural similarity functional group and chain length. Hence, common functional group or substructure (e.g., Aminoalkyl ethers) present in both chemical moiety of N-Methyl-N-nitrosoethanolamine as well as N-nitroso Orphenadrine impurity
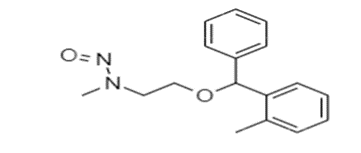
N-Nitroso Orphenadrine
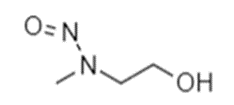
N-Methyl-N-nitrosoethanolamine
Data from surrogate chemical on either side of N-Methyl-N-nitrosoethanolamine can to be used of an TD 50 value as 1.29 (mg/kg/day) (Koepke SR et at., Cancer Res. 1988 Mar 15; 48(6):1533-6) to predict the Acceptable intake limit 1290ng/day for N-nitroso Orphenadrine impurity.
Lhasa Limited Carcinogenicity Database
That looks about right - I think there’s a few places where “any except hydrogen” may apply, where you currently have a carbon (such as the top row of the aryls), but that might just muddy the waters…
That study certainly supports arguments for a limit higher than 18 ng/day, but looking into the details of it I suspect it is not sufficient by itself which would be why it wasn’t used.
- Single dose plus control
- Short study duration with respect to lifetime
- Only 20 male rats per dose group
In other words, there are several significant issues with the study. The lower CI is 0.522 mg/kg/day, which may be able to be argued for, and it should be noted that at the administered dose less than 50% of rats had tumours.
Watch out for an upcoming paper in RTP (Felter et al) where we dig into how to make use of these less-than-perfect studies in more detail.
Separately, the proposed analogue is a beta-hydroxy which is known to be potency-reducing, whereas in nitroso-orphenadrine the hydroxy has an aryl group on it which will significantly change the nature thereof, so the read-across may need additional justification.
Certainly, it’s a bubbling of ideas! That’s how science works, that’s how science thrives and evolves. @Naiffer_Host , the gratitude is all mine for the opportunity to be part of the community and contribute to this important discussion.
Excellent summary! Clear, concise, direct, and very informative.
Dear all,
how do you interpret the bold text in the last paragraph on page 27?
The Carcinogenic Potency Categorization Approach applies to N-nitrosamines bearing a carbon atom on both sides of the N-nitroso group, and where the carbon is not directly double bonded to a heteroatom (i.e., N-nitrosamides, N-nitrosoureas, N-nitrosoguanidines and other related structures are excluded). Additionally, the potency categorization approach does not apply to Nnitrosamines where the N-nitroso group is within an aromatic ring (e.g., nitrosated indole). For Nnitrosamines containing two N-nitroso groups, the group with the highest predicted carcinogenic potency (i.e., the group with the lowest numerical potency category) defines the AI for the entire molecule.18 The α- and β-carbons are defined relative to the N-nitroso group, as illustrated in Figure 1.
Is the CPCA not applicable here and we have to use 18ng/day as AI, or are nitrosamines based on these structures not to be considered at all? This would make life quite easy for all potential imidazole-based nitrosamines.
This has the potential to be very positive for the industry but only if the FDA and Health Canada follow suit. Is there any information to indicate that the FDA and Health Canada will align their guidance to Rev.16 update of the EMA guidance?
Fantastic thank you for this!