Please refer to these links for aryl definition:
Aryl should have a benzen ring and that’s not the case I think here. Either ways, both scores of 1 and 0 will imply 18 ng/day limit. Really thanks for your help and extended support
Please refer to these links for aryl definition:
Aryl should have a benzen ring and that’s not the case I think here. Either ways, both scores of 1 and 0 will imply 18 ng/day limit. Really thanks for your help and extended support
Engaging discourse on the subject of organic chemistry!
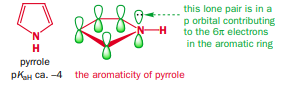
@Alaaelkazak, within the pyrrole molecule, the lone pair of electrons residing on the nitrogen atom effectively contributes to the count of six π electrons required for the establishment of aromaticity within the system, as you can see here:
Why FDA set limit of 96 ng/day for N-nitroso-vonoprazan, any other scientific evaluation is there.
Moreover we can said there is again different approaches regulatory to regulatory.
I’m afraid that link is incorrect, not only in comparison to what I was taught (my undergrad was organic chemistry) but also specifically in the context of how the term aryl is used in the nitrosamine SAR work - we have used both in discourse and the underlying patterns, (which simply require an aromatic atom there, see below) the term to mean all aromatic systems. As an aside, the strength of aromatic systems can vary, in that some are more effectively-delocalised than others, but we’ve considered essentially anything that obeys Huckel’s rule - as @lucas10mauriz has explained this does - or more precisely anything accepted by the computational representation, whether SMARTS or any internal representation tool, as aromatic. In practical terms for the underlying patterns, this means that the SMARTS definition of this feature will be something along the lines of aCNN=O or cCNN=O.
To further confusion, we have referred to this occasionally as a benzylic-like a-carbon, which does formally imply c1ccccc1CNN=O, but that is simply because the word arylic doesn’t exist, and we’ve always made the effort to clarify that we do mean all aromatic systems in this case.
A limit of 96 is almost certainly derived by read-across from NDMA, which can be at least somewhat justified for any N-methyl-N-nitroso group (though there’s normally a more precise analogue than this). Remember that the FDA have not (yet? my understanding is that there has been regulatory collaboration on this so we might see changes in future) published the CPCA, so the approach is currently formally only valid for EMA submissions and any health authority that has specifically written their guidance as “copy EMA exactly”.
The way the EMA guidance is written, the CPCA should be performed first, but can be over-ruled with robust data, either a robust read-across or experimental toxicity data.
That’s really interesting explanation! Don’t you think that a glossary or definition list should accompany such guidelines to ensure eliminating any misunderstanding or misinterpretation?
I think “aryl” and “aromatic” are generally understood as described - especially when looking at the examples in the guidance and the limits for those compounds that have CPCA limits… in terms of a specific glossary, I suspect we’re out of luck due to the decision to refer the electron-withdrawing group (EWG) definitions back to Cross & Ponting rather than enumerating them.
My general thought would be to ask here (see for example the discussions of beta-methyl groups in another thread) if there are any features that the precise definitions of which are unclear - for example, I can state with some confidence that the EWG list, though there are many other groups that do withdraw electrons, is pattern-able as “*A=,#Q, *CF3, not *C([C,H])=O and not *COOH”.
@Yosukemino is there a calculation here for Sitagliptin NTTP?
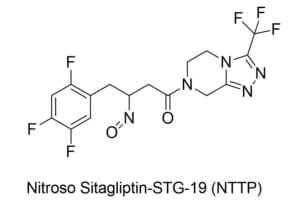
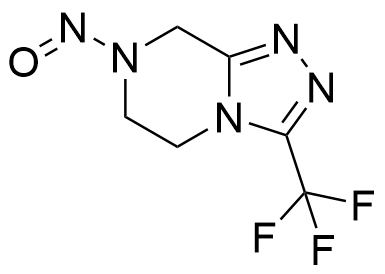
@Yosukemino i think this structure is the correct one
https://www.tga.gov.au/news/safety-alerts/sitagliptin#:~:text=7-nitroso-3-(trifluoromethyl)-5%2C6%2C7%2C8-tetrahydro[1%2C2%2C4]triazolo-[4%2C3-%20a]pyrazine.
that is I believe the structure - it’s not nitroso-sitagliptin itself, rather the nitrosamine of an impurity of sitagliptin.
It has been assigned a compound-specific limit of 37 ng/day by read-across from nitroso-1,2,3,6-tetrahydropyridine (with the aromatic group assumed to be comparable to the double bond there and the ‘arylic’ position like the allylic in NTHP), though by CPCA would have a limit of 100:
(2,2) for H-count = 1
In a generic 6-membered ring = 2
Aromatic group at b-position activating it = -1
Total 2, limit 100 ng/day
So in those case, what overrule… the smallest AI or the CPCA AI limit?
@David Thanks for clarity on subject.
However still question is that, which approach for determination AI is suitable and valid, either SAR or CPCA and how it will be decided.
My understanding is that in this case, because there is a defined limit, that takes precedence, however in the general case CPCA should be tried first but can be overridden if needed (and it will be - there are a lot of nitrosamines still in categories 1-3!) by read-across.
Can anybody tell about the logic of this alpha H atom given in table regarding the score
Correct David, Similarly sore 1 have 18 ng/day whereas US guidance tell about 26.5 ng/day for worst case scenario.
Score of N-nitroso-vonoprazan is 1 = 18ng/day
And David, do you think that the double bond is a really suitable analogue for the aromatic ring? The CF3 is a strong electron withdrawing group that can certainly render the aromatic ring with very unique electronic properties that would be have an impact on the alpha hydrogen of the nitrosamine, and it would be very different from the double bond.
In fact, while it cannot be categorized currently as an EWG, it may have that effect. Do you think that modelling softwares could predict if this would be the case?
Javier
Dear @ASrinivasan, thank you very much for the reply!
How do you see this issue:
Additionally, the potency categorization approach does not apply to Nitrosamines where the N-nitroso group is within an aromatic ring (e.g., nitrosated indole).
Is the CPCA not applicable here and we have to use 18ng/day as AI, or are nitrosamines based on these structures not to be considered at all? This would make life quite easy for all potential imidazole-based nitrosamines.
For the nitrosated indoles, they aren’t nitrosamines per se; the indole nitrogen is not a secondary amine. Definitely still worth mentioning in the assessment if you consider there is risk of formation or have evidence thereof (don’t conceal anything is normally good advice!), but the implausibility of diazonium formation means that I would argue very strongly for treating these as conventional impurities under ICH M7 rather than as part of the cohort of concern.
I recently summarised this on another thread: 🗓 Nitrosamines Conversation Event w/ Dr. Raphael Nudelman - #13 by David
We are asked to test 3-methyl-1-nitrosoindoline, 1-nitroso-indoline and 2-methyl-1-nitroso-indole as a part of nitrosamine risk assessment by Health Canada in recent query.