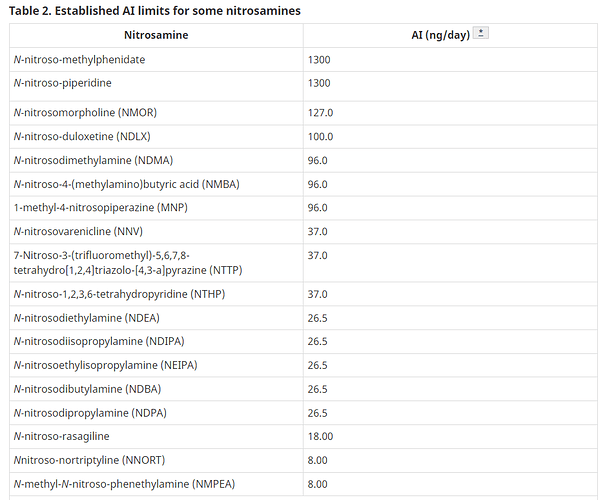
Thank you for sharing the information, @Rafa. The limits list by Health Canada is useful because it includes the limit of NDSRI such as N -nitroso-methylphenidate, N -nitroso-duloxetine, N- nitroso-varenicline, N -nitroso-rasagiline and N nitroso-nortriptyline. And the limit of NDSRIs is considered to be calculated from those of nitrosamine with the same AI. This list is helpful to determine the limit of other nitrosamines!!
Thank you for the information of Health Canada ! The limits of NNORT and NMPEA are surprisingly low compared to NDMA or other major nitrosamines. I’m interested in the reason.
Hi, @Makimura. You can find the toxicity data of NMPEA in LCDB . The lowest TD 50 is 0.00797 mg/kg/day. And the limit of NNORT is probably calculated from that of NMPEA.
Good evening, @Yosukemino . I was able to understand the calculation . Thanks for your kind reply.
Thanks @Rafa for uploading the information. I do appreciate @Yosukemino and @Makimura for raising the question about low limits for NNORT and NMPEA.
@kpcross your insight is always welcome and appreciated!
Hello,
Today EMA updated their list of limits as well and guidance on Ames tests was added.
Thank you for sharing the update, @pagelm. Rev.9 includes limits of seven new nitrosamines. The updated list is almost the same as Health Canada’s, as it does not include N-nitroso-duloxetine.

For Ames tests, a new clarification is added.
- Taking these concerns into account, a well conducted GLP-compliant in vitro bacterial reverse mutation test performed according to the OECD Guideline 471 and the concerns above can be used as part of a weight of evidence approach, but additional supporting evidence would be required to classify a nitrosamine as a Class 5 impurity.
We should keep it in mind.
Thank you very much @pagelm and @Yosukemino for sharing.
Hi, Thanks for sharing…
Hi, Thanks for sharing…
Thanks for the information. 
Could you tell me the reference of the limitation of N-nitrosopiperazine of FDA? Thank you very much.
Dear Naiffer,
In the recent past I had to work on analytical procedures to determine the content of 7-Nitroso-3-(trifluoromethyl)-5,6,7,8-tetrahydro[1,2,4]triazolo-[4,3- a]pyrazine (NTTP), which is present in the list you posted as per Canada Health. Its AI has been recently published in the following EMA document with the same limit of 37 ng/day.
Thanks @Dan for the update. I have included it in the table. Would you mind sharing with the community details of the methodology?
Hi Naiffer Sir, I need recent published values from ANVISA’s Nitrosamine guideline along with China published guideline value.
Could you please share the same.
Thanks
Nilesh
@Nilesh I tried to keep the table as updated as possible with the information that members share in this post.
May I suggest to connect with @jxl for clarity on China’s published values or @PauloEliandro for recent ANVISA’s values.
Great Sir…
It would be really great if you connected me today or at this moment to both of them for China & ANVISA updated guidance value.
If you share recent guidance’s for both China & ANVISA, that is also fine for me
Dear sir,
Kindly share the updated FDA AI limit also, as unable to trace out in FDA guidance’s website, the recent one…
We have a category ‘Guidances’ where members of the community publish them. Happy browsing