@Naiffer_Host Please add 4-(methylnitrosamino)-1-(3-pyridyl)-1-(butanone) (NNK) AI 100 ng/day from Health Canada Guidance update from -01 September 2022 at https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-health-products/compliance-enforcement/information-health-product/drugs/nitrosamine-impurities/medications-guidance/guidance-nitrosamine-impurities-medications-eng.pdf
@pragnesh
Thanks for sharing this information
@Naiffer_Host @pragnesh @Yosukemino @jxl @Sarada.jena @Rafa
Dear Team,
Moreover, as per recent updated guidance’s regarding Nitrosamine impurities, 3 new Nitrosamine AI limit has been published.
- 4-(methylnitrosamino)-1-(3-pyridyl)-1-(butanone) (NNK) - 100ng/day
- N-nitroso-dabigatran- 18.0 ng/day
- N-nitroso-tamsulosin- 18.0 ng/day
guidance-nitrosamine-impurities-medications-eng.pdf (canada.ca)
However, 4-(methylnitrosamino)-1-(3-pyridyl)-1-(butanone) (NNK) is new Nitrosamine Impurity AI limit is published in Health Canada guidance’s only.
N-nitroso-dabigatran impurity AI limit is published in EMA & Health Canada guidance’s only, having same 18.0 ng/day AI limit.
N-nitroso-tamsulosin AI limit is published in TGA & Health Canada guidance’s only, having same 18.0 ng/day AI limit.
Thanks
Nilesh
Sir, US FDA used lowest TD50 value of 0.0374 mg/kg/day from N-Nitroso-1,2,3,6-tetrahydropyridine.
Dear all,
Yesterday EMA published Rev 12 of the Q&A nitrosamines guideline. In particular:
Newly added to Q&As 10 and the list of potential N-nitrosamines is
4-(Methylnitrosoamino)-1-(3-pyridinyl)-1-butanone (NNK) with a limit of 100 ng/day and
N-nitrosoduloxetine using a limit of 100 ng/day.
Furthermore, the footnotes for N-nitrosoduloxetine was added clarifying that the limit derived using structure-activity-relationship (SAR) /read-across approach using the TD50 of NNK as point of departure.
Additionally, provision has been incorporated to allow the marketed products with a limit of 178ng/day whose AI is not yet established and the levels are found above 18ng/day. This means if the level of the nitrosamines found above 18ng/day, agency will not trigger any market action (product recall) for the period of 12 months or until the AI is established, whichever is earlier.
I am assuming that it is not applicable to product under development or during on-going MAA applications. For such cases, still Q&A 10 should apply.
Your thoughts are welcome.
Thank you for the update. NNK and N-nitrosoduloxetine AI Vales are similar to the health canada.
@Yosukemino @pragnesh @Naiffer_Host
Dear team,
Could you please share me the AI limit for N-nitroso fluoxetine impurity and where its AI limit in published.
Thanks
Nilesh
There is no official limit published for Nitroso Fluoxetine. But the limit can be derived using structure-activity-relationship (SAR) /read-across approach using the TD50 of NNK as point of departure same as Nitroso Duloxetine.
Thanks for your reply, sir…
Could you please explain more in detail for AI limit for the same?
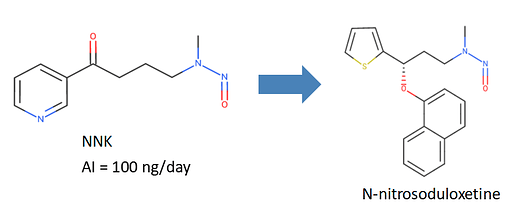
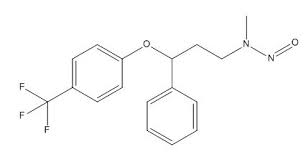
Based on above chemical similarity, AI for N-nitroso fluoxetine can be proposed as 100ng/day.
@Yosukemino @Naiffer_Host @Diego_HM can you please provide input and guidance
There is no published regulatory limit yet on n-nitroso fluoxetine. So unless we are using nitrosamine class specific US or EMA TTC values, a thorough literature search analysis work on analogues is needed to propose suitable analogue based AI for n-nitroso fluoxetine.
@pragnesh
Thank you very much for your reply
Above interim limit justification was mentioned in EMA guideline of version 21 December 2022
EMA/409815/2020 Rev 14
Requesting provide input Regarding above reference how 13.3 value is considered in 13.3XAI ??? How limit will be calculated based on duration treatment??.
Dear @vinod.thorat- The section you are referring is LTL approach. To use LTL, approach, first an AI should be available .
Now based on maximum duration of treatment expected for your drug with indication- In case if the duration of treatment is within 1 year- Multiply the already available AI with 13.3.
Suggestion: Before using this, appraise the interim limit with lead-Authority or National Competent Authority (Europe).
Thanks for kindly revert
Kindly share the update list
Thanks.
Hi, everyone, is proposed limit available for N-Nitroso-methylpropylamine?**
Hi Robin,
Most likely you will use read-across to define your AI…
Additionally, the carcinogenicity Database reports as positive carcinogen

