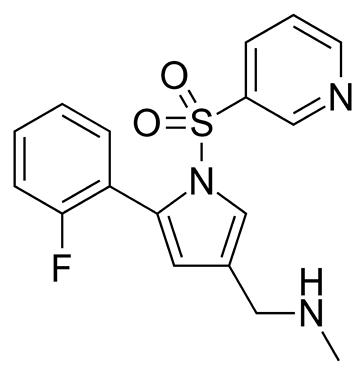
Phathom Pharmaceuticals reported that it detected trace levels of a nitrosamine impurity (may be NDSRI, nitroso-Vonoprazan ?? ) that is not described within the FDA guidance document entitled “Control of Nitrosamine Impurities in Human Drugs – Guidance for Industry.” The Company is working with the FDA and plans to obtain approval of and implement an additional test method, specification, including a proposed acceptable intake limit, and additional controls to address this impurity prior to releasing their first vonoprazan-based products to the market. These additional activities will result in a delay of the planned VOQUEZNA DUAL PAK and VOQUEZNA TRIPLE PAK product launches. The Company currently expects the full commercial launch of these products, as well as, if approved VOQUEZNA tablets for EE, in the first quarter of 2023.
This is obviously a secondary amine and this represents a high risk in terms of forming the NDSRI - NO-Vonoprazin. The challenge as with so many similar examples is what would be an appropriate AI. Illustrates again the need to have an effective interim position in terms of limits.
日本上市的样品我们检过,检出量1ppm,而且稳定性样品会显著增加
该亚硝胺的控制限度,控制在什么水平呢?什么时候会有控制限度的相关要求 出来?
这个杂质有控制限度了吗?目前可参考什么制订限度?
Either,
(1) you can use nitrosamine TTC value
US - 26.5 ng/day
EU - 18 ng/day
(2) Derive AI based on read across chemical with the help of qualified toxicologist
原研检出量大于1ppm,用这两个TTC的控制限度,都控不住。需要提高控制限度,EMA在2022年10月发布的问答文件
EMA亚硝胺问答文件-409815-20221013-英文-12版.pdf (591.5 KB)
(409815,12版)中,提出了178ng/天的总亚硝胺控制限度,是不是可以采用呢?
I think, the decision whether to use TTC or 178 ng/day will be taken by regulators (FDA, EMA or PMDA) based on drug supply shortage possibility for the medicine involved. Also, if qualified toxicologist can justify the data using read across which support higher limit than TTC or 178 ng/day, it can be proposed as well.
The limit of N-nitroso-vonoprazan (NVP) is considered as 96ng/day which is the same as NDMA, according to this article.
由API与辅料中的亚硝酸反应产生,同时稳定性放置过程中会持续增长,加速6个月可超出控制限度。[quote=“Naiffer_Host, post:12, topic:3278, full:true”]
@Xuguoqin would you be able to share more about how the impurity increase during storage?
[/quote]
According to the following article, Phathom Pharma has already succeeded in mitigation measures.
Following a meeting with the FDA, the company said that they undertook mitigation measures, including a minor tablet reformulation, which will stop the growth of the NVP. This data was shared with the FDA along with stability data, and the FDA provided feedback on the expected stability data needed. Based on that feedback, the company expects to refile in Q2, and with a probable approval and launch in both indications in Q4.
So it was a stability issue, but does anyone know if it was the stability of the FDF or the API itself?
Phathom Pharmaceuticals Resubmits Erosive GERD New Drug Application to FDA
By Globe Newswire May 23, 2023
- Phathom Pharmaceuticals resubmitted its New Drug Application (NDA) for vonoprazan.
- Resubmission responds to the Complete Response Letter (CRL) issued by the FDA in February 2023 relating to specifications and controls for a nitrosamine drug substance related impurity, N-nitroso-vonoprazan (NVP)
- As previously communicated, Phathom implemented mitigation measures, including a minor drug product tablet reformulation, to inhibit the growth of NVP, and has been conducting a stability program to demonstrate these measures are effective and support the commercial shelf life.
- The NDA resubmission contains three months of stability data for six batches of the reformulated vonoprazan tablets. The three-month data demonstrate that Phathom’s mitigation measures are controlling NVP growth through three months and keeping levels well below the acceptable daily intake limit of 96 ng/day or 2.4 ppm (parts per million) based on the maximum approved daily dose of 40 mg/day. All the manufactured reformulated batches have demonstrated control of NVP through three months of long-term stability conditions that are more than tenfold below the acceptable intake limit and which Phathom believes support the requested shelf life of 24 months based on its statistical modeling.
- As agreed with the Agency, company plan to provide the final six-month stability data during their review as it becomes available. The data we have collected so far, and our statistical modeling, reinforce our confidence that the reformulated vonoprazan tablets comfortably support the 24-month shelf life we originally requested.
24 month shelf life proposed based on statistical model