They future problem, and I think it was referenced, is data sharing. If I understand correctly, If Companies A & B have competing products, each would need to bring their own data, slowing down the process & increasing costs. I understand that Company B should not just be given the data, but we need better answers.
dear all,
i have participated in a few sessions due to the time difference.
I want to stabd to the two presentations regarding the NDSRIs from impurities and the conversion rates and their relation to the nitrosamine formation risk.
In three cases, the conversion rate was not more than 0.24%. This low rate it is anticipated due to low impurity content in the final product.
It would be very useful and interesting the design of a similar study in a bigger scale in order to identify a ‘‘space’’ and if possible an upper limit of a plausible conversion, e.g. 0.5%. This would be a great tool for the preparation of risk assessment reports.
thanks
Christos
@chrischar - it was an interesting presentation with quite a lot to take in.
One thing I wasn’t sure about was whether these were manufacturing impurities, or whether they were degradation products of the API that could form over time? So were the rates of reaction just due to the low level of initial “contamination” with the impurity being a manufacturing impurity, or were they driven by the rate of formation of the degradation product in the product over time.
They may have explained this, there was an awful lot to take in through the 2 days when attending virtually.
good point Mark,
to be honest i did not notice any clarification on this.
But you are right, a lot of degradation impurities are process related as well.
In addition, i think that the very useful equation for the risk evaluation
(quantity x conversion x MDD)/AI
should be compared to 0.5 and not to 1 for established level of risk, in order to include in our evaluation and the plausible increase due to stabilit.
thanks
I have to admit that conversion rates are a powerful data set that will alleviate A LOT of the suffering… FDA and other agencies sit on that data based on all the results reported by manufacturers; unfortunately, the FDA can not use that data for confidentiality reasons.
That being said, good trends could be drawn if companies were willing to blindly share their conversion or reactivity rates with a third party. Taking @chrischar’s comment on a large-scale study, an enhanced NAP test could be a good surrogate of API conversion. It’s not ideal because it’s just the API, but at least it gives the worst-case scenario. thought?
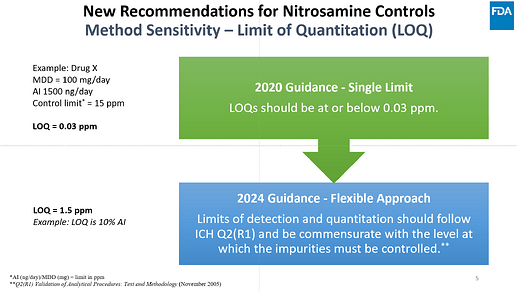
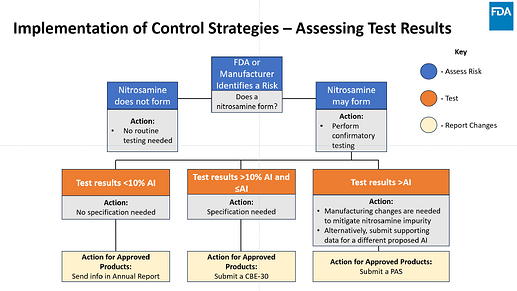
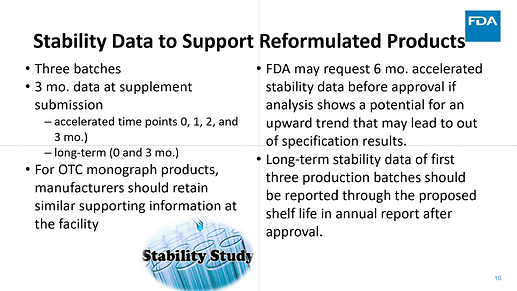
I want to share few slides from a couple presentations made by FDA that can help clarify many of the questions that we received here in the community post FDA revision (Sep 2024) :
How to calculate the limits with more than one nitrosamines (by Dr. Dan Berger)
Implementation of Controls Strategies (by Dr. Susan Zuk and Dr. Dongmei Lu)
Dear Naiffer,
i do not think that NAP test is suitable for the case of the NDSRIs of the impurities.
As we saw form the cases which have been presented by Jingyue Yang and Xiang Yu, the conversion rates (CR) were less than 0.5% in all cases, while in NAP tests the CRs are even more than 50%.
So, with NAP test may be you have the worst case but taking this into consideration in the risk assessment you may overestimate the risk about 100 times!!
In my mind the suitable study for this, should be focus on real cases of stability results of NDSRIs of impurities from several final products in order to have a ‘‘library’’ of conversion rates and from there to adopt the worst value.
hope this is clear,
Christos
I agree that the test will provide an overestimate, but at least it provide some level of information vs none. I also agree that a specific formulated product conversion will need to follow and be study for the particular formulation. I am coming from the general knowledge perspective.
@jason.brown presented an excellent study at AAPS that examines a specific formulation conversion, but that would need to be carried out for every formulation.
Similarly, the modeling that Dr. Ian presented at the CRCG-FDA workshop, while the reactivity modeling provides an overestimation, is a valuable piece of information as risk assessment is undergoing.
unfortunately i missed the presentation of Dr Ian due to the time difference.
For sure i will watch it when the recorder version of the workshop is available.
thanks for sharing your thoughts,
christos
Worth attending both days.
Could you please share all workshop presentation for Updates on Approaches to Acceptable Intakes of Nitrosamine Drug Substance Related Impurities (NDSRIs) and Bioequivalence Assessment for Reformulated Drug Products
By first half of December the presentations are expected on the Youtube channel of CRCG.
Now recordings and slides are available. Please check the event page.
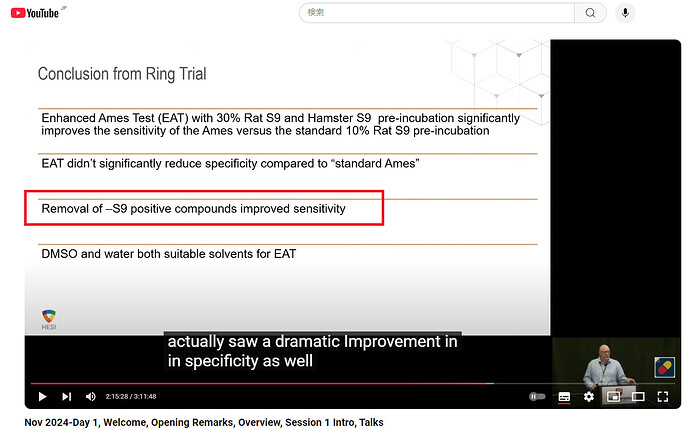
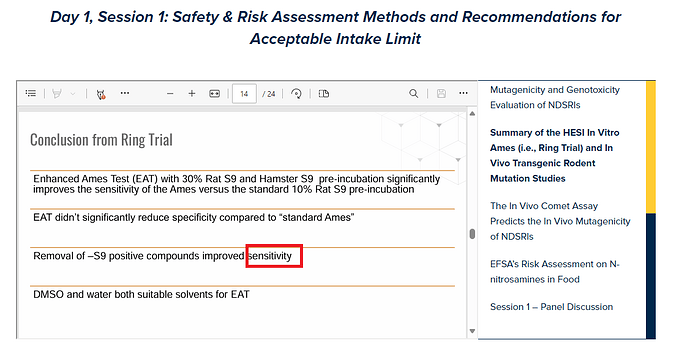
Thank you for the fantastic presentation, @jbercu. I want to confirm that you stated that the “specificity” also improved dramatically when -S9 positive compounds were removed in the ring trial. Is that correct? It is interesting. Direct mutagens should be distinguished from nitrosamines.
yes that was a typo, unfortunately the slides could not be changed. However, I changed to specificity in the downloaded slides.
Thank you for the additional information. However, downloaded slide was not corrected. I appreciate your help. ![]()
The Ames Ring Trial literature is now publicly available. Thank you, @jbercu, that’s great!
HESI GTTC Ring Trial: Concordance between Ames and Rodent Carcinogenicity Outcomes for N-Nitrosamines (NAs) with Rat and Hamster Metabolic Conditions
HESI Ames Ring Trial Analysis
https://hesiglobal.org/DEV_t_concordance_v5.1.html
Conclusions
Based on the outcome of the study, 30% hamster and 30% rat liver induced S9 (Combination 8) had the highest sensitivity (93%) for predicting rodent carcinogenicity compared to all other conditions, with little impact on overall specificity. For higher molecular weight NA’s, DMSO was shown to be a suitable alternative to water as a solvent since it has little impact on the overall outcome of the Ames test. Between laboratory reproducibility was also high when using 30% hamster and 30% rat liver induced S9 (91%), indicating a high degree of transferability for the method. Inconsistent results were observed with sterically hindered NAs that may show limited metabolic activation and / or are less potent rodent carcinogens. Nonetheless, these results support the use of 30% hamster and 30% rat liver induced S9 (EAT guidance) for identifying carcinogenic NAs using the Ames test.
Thank you @Yosukemino. It was a team effort. It shows the Enhanced Ames Test is a very sensitive assay! If people have questions please let me know.
looks like they didn’t update the slide, my apologies.
Thank you @jbercu. This is a great publication. From supplemental data, I found that most nitrosamines with negative carcinogenicity have insufficient data. We should pay attention when dealing with the specificity of nitrosamines.