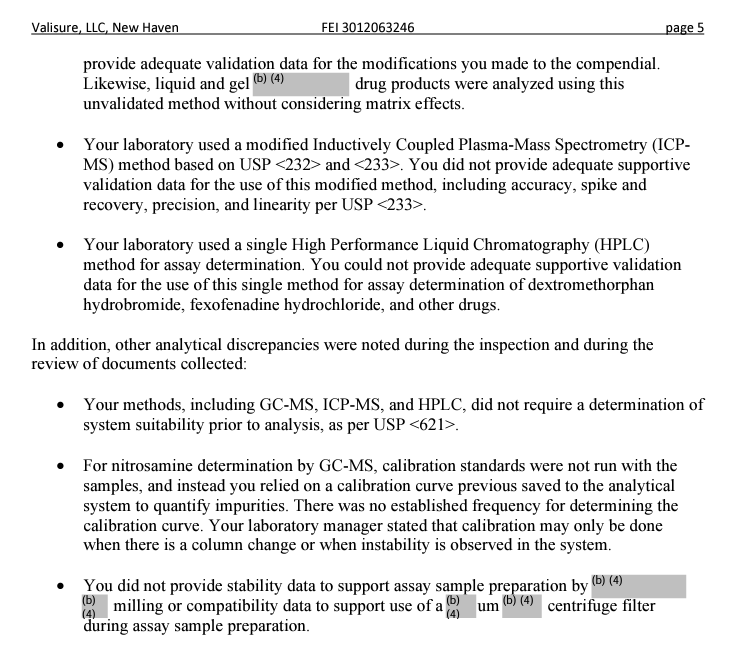
In a Dec. 5 letter, the agency wrote Valisure that its online pharmacy failed to take several basic steps to ensure that suspect or illegitimate medicines were investigated and that distributors were alerted. Moreover,the FDA cited its laboratory for “methodological deficiencies” in testing medicines over concerns that the results may dissuade its customers from purchasing certain medicines.
2 Likes
Regulatory Focus published an article for this alert. The Valisure tested only for informational purposes, not for any regulatory purpose.
1 Like
Is this the same company whose methodology was criticized in the recent Zantac judgement MDL NO. 2924?
1 Like
You are right…its same Valisure
1 Like