To be honest, I had never considered the geographical location of the factory as a root cause, but I’ll let you all read…
NOx risk and factory location links I have not seen explained yet to local air quality (this is a first) but in some cases to the accessibility of high quality natural gas and thus the usability of direct heating processes.
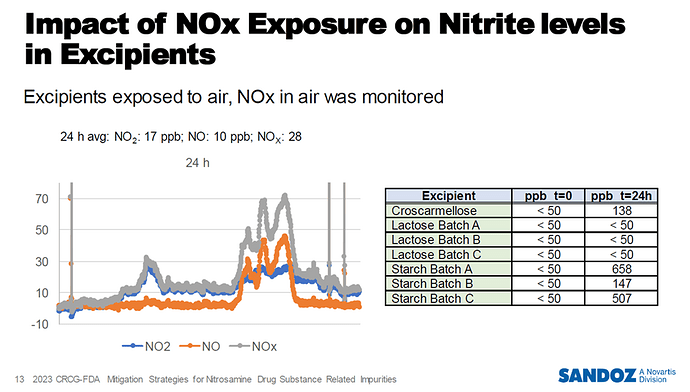
I remember also the presentation of Dr. Rok Grahek:
It’s a great publication!! One of the authors explained that they would change the factory from Osaka to Yamagata for DP(he did not say it was metformin then) production at the symposium in Japan. It was difficult for them to remove NO2 from air by trapping something. And nitrogen gas was not available for fluidized bed granulator due to the large production scale.
I heard the same thing happened to a different API in a different company. We should pay attention to NO2 in the air!!
@Yosukemino, as we uncover possible root causes, we often find ourselves surprised by the possibilities…
I learned today about a research effort to quantify the amount of NDSRI formed from potential exposures to NOx… Can’t wait for the study to be published!
its not just the atmospheric air. Spray drying is quite common to provide materials as a powder. The air is often directly heated by a natural gas burner so the exhaust contacts the product…if the burner is not optimised NOx can be formed. There is a lot of information about this in thee food industry as they have known about the issue for 40 odd years.
Intriguing! One more area to be focused on as a part of control during process. This obviously pose a challenge during analysis by GC-MS -head space technique if air contains NOx. My two cents thought.
That’s a fantastic perspective. Can you point out a reference publication that explores that topic for us to dive little deeper ? Thx
The brewing industry had a lot of info on removing direct heat from any processes if I remember correctly - especially the malting process - so as not to expose to the gas burners that were used previously.
Sure, this is probably the best i have found.
Formation and occurrence of nitrosamines in food - PubMed (nih.gov)
Very valuable points.
I was also pointing to this in my original comment (“NOx risk and factory location links I have not seen explained yet to local air quality (this is a first) but in some cases to the accessibility of high quality natural gas and thus the usability of direct heating processes.”)
This is also an interesting summary:
Direct vs Indirect Fired Heaters for Spray Dryers – GLM Hydro
Not all drying processes would avoid contact between the NOx and the excipient undergoing drying and I agree specifically spray drying processes could indeed make use of either direct or indirect fired heaters, with indirect fired heaters (for example by using a heat exchanger) having the benefit of heating the air indirectly, meaning that the to be dried product is not exposed to exhausts from the burner and thus NOx resulting in contamination in the excipient.
Over the last years I have been discussing a lot on direct vs. indirect heating processes, upon checking if spray drying is used, as it is in my experience the most common root cause for high nitrite excipients (especially when final excipients are higher in nitrite than their native raw material that was used).
It is interesting that mixed positions exist here in manufacturing practice (and this has sometimes again a link with the geographical location, due to the accessibility of high quality natural gas), some manufacturing experts judge that using direct heating for spray drying processes is only suitable for feed, and has long been removed for food and pharma production (coinciding with the cited interest on this topic in the food industry for already many years), while other companies still find it is acceptable for pharma excipients (and ignore the nitrite contamination and resulting nitrosamine issues this gives, as long as the daily intake of nitrite as such isn’t too high compared to EFSA recommendations) and while other companies manage to get fairly low nitrite contamination with direct heating processes as they do with indirect heating processes as long as the process is under control and high quality natural gas is available in the region (in India it appears to often not be available).
In the end both spray and bulk drying and both direct and indirect heating processes can be designed to yield low nitrite excipients, but it will be interesting to see with the advancing and finetuning of nitrite testing techniques if very low LOQ methods will allow further distinctions between process capabilities. The key hurdle is mostly wanting to design a low nitrite process (and apply the necessary controls).
The new metformin paper but also the slide from Rok Grahek are valuable materials to guide discussions on NOx contamination being a real risk. Historically nobody debated that NOx can be a nitrosamine formation risk as there are quite some literature contributions on this, point of discussion is often on if a certain process is sufficiently lowering NOx contamination risks in so far that no testing is needed. I see that with (spray) drying of excipients, but also with the industrial manufacturing of simple amines and how scrubbers and torches are implemented.
This is very well known in the world of nitrosamines. The sad part is that all that knowledge is being replaced by pseudo science of reinventing of wheels. If you read the very first white paper of AAM in 2020, of which I was one of the authors (https://accessiblemeds.org/sites/default/files/2020-10/AAM-Nitrosamines-White-Paper.pdf) I mention NOx. My work in NCI was on nitric oxide (which was then the superhero of chemistry). But one issue is that nitric oxide reacts with oxygen to produce NOx that can cause nitrosation. In many meetings I mentioned a crude experiment that I did during my PhD, where I took some cream (most creams contain triethanolamine or trolamine) in a petridish and kept it in a closed system, where I injected some nitric oxide. After 2 days, I saw a significant rise in the levels of NDELA. In my talks I have used this to establish two points. Tertiary amines nitrosate in presence of NOx in a facile manner and NOx is a major source of nitrosating agent. Coming from Kolkata in India and having in-laws in Mumbai, I always joked about how much NOx I take everyday when I visit ![]()
Again “NEW”? Read this paper by Challis, published in 1978
“Challis BC, Edwards A, Hunma RR, Kyrtopoulos SA, Outram JR. Rapid formation of N-nitrosamines from nitrogen oxides under neutral and alkaline conditions. IARC Sci Publ (1971). 1978;(19):127-42. PMID: 28274.”
Also, Keefer and Saavedra have several papers on NOx and nitrosamines.
Another old example:
What does feel quite new (or at least not as common practice) though is the broadening of the attention to NOx monitoring in pharmaceutical manufacturing practice, thanks to this and other new contributions.
I don’t think many are doing or are easily convinced to doing NOx detections in root cause investigations or as step 3 (mobile gas detectors for spot checks or a continuous nitrogen oxides analyzer linked to a specific process element as PAT), partially also linked to equipment availability.
From that perspective newer literature to make points really helps.