Hello to everyone!
Is anyone aware of Nitroso Duloxetine AI? I came across with an AI of 100ng/day. Is there any literature available?
Thank you,
Elena
Hello to everyone!
Is anyone aware of Nitroso Duloxetine AI? I came across with an AI of 100ng/day. Is there any literature available?
Thank you,
Elena
@elenaly can you share a bit more about this… what was the source of the AI, and do you know of any alert on this product?
Hello Elena,
Yes, a compound specific acceptable intake (AI) limit of 100 ng/day has been recommended by Health Canada.
From my correspondence with the agency, it seems that Health Canada’s acceptable intake (AI) limit for N-nitroso-duloxetine was derived following a structure-activity relationship assessment that identified 4-(methylnitrosamino)-1-(3-pyridyl)-1-(butanone) (NNK, CAS: 64091-91-4) as the analogue for read-across.
Hope this helps.
Best wishes,
Dear @Naiffer_Host ,
Our Duloxetine main supplier informed us about this AI for Nitroso-Duloxetine, but still we are in the beginning of the discussion with them, so we don’t have the explanation behind the AI yet.
It seems that Health Canada is ahead of FDA & EMA in Nitroso-APIs issue. But since we are based in Europe we must wait for EMA’s feedback.
Actually, EMA is catching up and I think it will be the same limit of the surrogate of 100 ng/day that @Muzaffar kindly shared with us. Is just a matter of time.
The surrogate shared is:
Lhasa Carcinogenicity Database - Study Information (lhasalimited.org)
N-nitroso-duloxetine
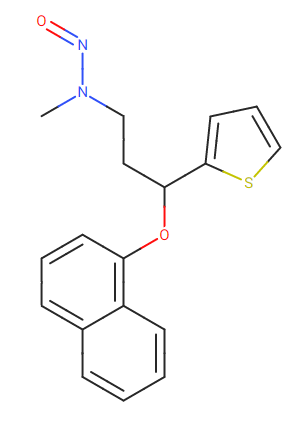
PROM Minutes 13 June 2022 (europa.eu)
Lhasa Carcinogenicity Database - Study Information (lhasalimited.org)
@Diego_HM @elenaly @Naiffer_Host @Muzaffar
Dear Team,
Moreover, TGA is also having same acceptable intake (AI) limit for N-nitroso-duloxetine i.e., 100ng/day
Thanks
Nilesh