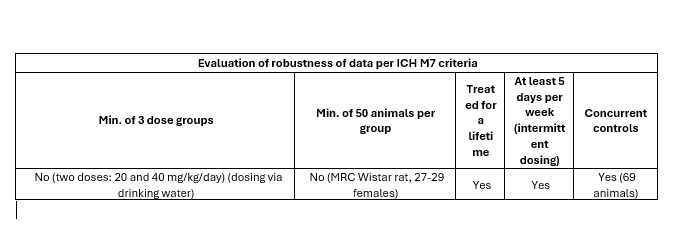
The Love 1977 study on 1-nitroso-piperazine is not meeting ICH M7(R2) robustness criteria and therefore a broader weight of evidence would likely be sought by regulators to derive a compound specific AI for 1-nitroso-piperazine.
There are also the historically observed TD50 trends within the nitrosopiperazine class (different substitutions) which are not always easy to explain (or were not always linked to the most robust studies) (and maybe elevate the burden on the need for a WoE).
Nonetheless, already in 1992, it has been argued by Lijinsky that mono-nitrosopiperazine might have reduced potency due to the basic properties of the remaining free nitrogen in the 6-membered ring and therefore can be different compared to the class as whole. (Meaning that the acceptance of a higher AI of nitroso-piperazine doesn’t per definition open the way for surrogate use for read-across with fully N-substituted NDSRIs, which is probably what you are thinking about rather than needing a limit for nitroso-piperazine itself).
Whereas 1-nitroso-piperazine was not one of the CPCA validation compounds of Kruhlak 2024, the data on this compound is discussed in the paper.
400 ng/day for 1-nitroso-piperazine (and others of the class) was an advancement, as originally read-across with small nitrosamines was applied for some nitroso-piperazines! For example, for 1-methyl-4-nitrosopiperazine EMA guided AI of 26.5 ng/day based on NDEA readacross (on 1 July 2023 revised to a 400 ng/day CPCA-based limit). Also the fact that there are no differences in CPCA workflow for piperidines compared to piperazines shows some indication that the designers tend to agree that nitrosopiperazines might not be typically and consistently more potent than nitrosopiperidines.
In Dobo 2022 structural group 10 consists of N-nitroso-piperazines and was assigned an overall AI of 153 ng/day based on the lowest robust TD50 in the structural group (1,2,6-trimethyl-4-nitrosopiperazine), suggesting that it would be prudent to assign an AI of 153 ng/day to the N-nitroso-piperazine class.
Bercu 2023 has endorsed this concept and evaluated a 150 ng/day limit for 1-methyl-4-nitrosopiperazine, while this nitrosamine is missing the beta-methyl substituent as present in the surrogate used.
dos Santos 2022 evaluates this to be a highly conservative limit based on a worst-case approach, considering that readacross with the most structurally related compound (N-nitroso-piperazine) would at least suggest an AI of around 5510 ng/day, while predicting that the AIs of N-nitroso-piperazines would typically range between 100 and 3000 ng/day.
The Bercu 2023 monographs also include an AI proposal for N-nitroso-piperazine of 28500 ng/day based on component-specific TD50 data (Love 1977).
Overall, for the N-nitroso-piperazine class TD50 data studied in the Dobo 2022 investigation, it became clear that the only alternative study having a similar robustness compared to the 1,2,6-trimethyl-4-nitrosopiperazine study (at least 2 dose groups, and at least 20 animals per group) was the Love study on 1-nitrosopiperazine.
It remains uncertain if the higher AI proposals (5510 ng/day, 28500 ng/day) will become accepted, but I’m not confident it is the way to surrogate use due to the free NH.
Another pragmatic approach could be applying 150 ng/day for N-nitroso-piperazines as a class and applying MW correction when deriving a limit for a NDSRI, which often will be just below 400 ng/day based on CPCA.
I suggest to start reading here:
Dobo, K. L., Kenyon, M. O., Dirat, O., Engel, M., Fleetwood, A., Martin, M., Mattano, S., Musso, A., McWilliams, J. C., Papanikolaou, A., Parris, P., Whritenour, J., Yu, S., & Kalgutkar, A. S. (2022). Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N‑Nitrosamine Impurities. Chemical Research in Toxicology, 35(3), 475–489. https://doi.org/10.1021/acs.chemrestox.1c00369.
Bercu, J. P., Masuda-Herrera, M., Trejo-Martin, A., Sura, P., Jolly, R., Kenyon, M., Thomas, R., Ponting, D. J., Snodin, D., Tuschl, G., Simon, S., De Vlieger, K., Hutchinson, R., Czich, A., Glowienke, S., Reddy, M. V., Johanssen, S., Vock, E., Claude, N., & Weaver, R. J. (2023). Acceptable intakes (AIs) for 11 small molecule N-nitrosamines (NAs). Regulatory Toxicology and Pharmacology, 142, 105415. Redirecting.
(For the monograph: see monograph 11 in SI)
dos Santos, C. E. M., Dorta, D. J., & de Oliveira, D. P. (2022). Setting limits for N-nitrosamines in drugs: A defined approach based on read-across and structure-activity relationship for N-nitrosopiperazine impurities. Regulatory Toxicology and Pharmacology, 136, 105288. Redirecting.
Love, L. A., Lijinsky, W., Keefer, L. K., Garcia, H. (1977). Chronic oral administration of 1-nitrosopiperazine at high doses to MRC rats. Zeitschrift für Krebsforschung und Klinische Onkologie, 89, 69-73.
Lijinsky, W. (1992). Chemistry and Biology of N-nitroso compounds. Cambridge Monographs on Cancer Research. Cambridge University Press, Cambridge and New York, 464 p.
Kruhlak, N. L., Schmidt, M., Froetschl, R., Graber, S., Haas, B., Horne, I., Horne, S., King, S. T., Koval, I. A., Kumaran, G., Langenkamp, A., McGovern, T. J., Peryea, T., Sanh, A., Siqueira Ferreira, A., van Aerts, L., Vespa, A., & Whomsley, R. (2024). Determining recommended acceptable intake limits for N-nitrosamine impurities in pharmaceuticals: Development and application of the Carcinogenic Potency Categorization Approach (CPCA). Regulatory Toxicology and Pharmacology, 150, 105640. Redirecting.