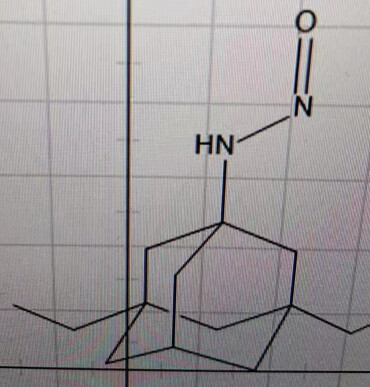
Is this an alerting structure, how to determine the AI? Thanks.
@jxl can you provide a bit more information on the inquiry?
Any guidance @kpcross @chakravarti_suman @David @MichaelBurns ? Thx
We’ve received this question from a DS manufacturer, they have identified this nitrosamine impurity in their manufacturing process, and it’s impossible to remove it because it’s a key intermediate. Since this structure is not covered by any guidance, they have to do the risk assessment. They are wondering if any research have been done on this structure to determine the AI. Thanks. -Jie
If you look at the presentation that I gave at the recent USP event (@Naiffer_Host - can you stick the link to the recordings in here)? you’ll see that I commented that primary nitrosamines may not be sufficiently stable in physiological conditions* to be considered as part of the cohort of concern. I’d suggest looking into that stability to confirm this statement, and attempt to suggest the general TTC (1.5 ug/day) on the strength of it (I know of no toxicity data at all on nitrosated primary amines, so cannot recommend any suitable readacross analogues, but also don’t think, as presented, they are cohort of concern). Additionally, the EMA in at least one version of the guidance drew the substructure of concern as explicitly a nitrosated secondary amine, so make of that what you will.
*This was based on some literature observations, including https://pubs.acs.org/doi/10.1021/acs.joc.0c02774 who state as part of the discussion of secondary nitrosamines that primary nitrosamines if formed are unstable, and also https://pubs.acs.org/doi/10.1021/ja9840807 and other work from these authors as to the lack of stability of primary nitrosamines (note: the nomenclature is a bit confusing; they discuss sec-diazonium ions, but these are formed from primary amines with e.g. isopropyl substituents)
Thanks David. What about Boc protected amine, it will be secondary nitrosamine if Boc group is there, does that make any difference on the stability.
Since there is no alpha-H in the structure, can we assume this nitrosamine will not be metabolized to generate alkylation species.
Thanks,
Jie
A boc-protected amine is functionally a carbamate; I would actually be more concerned about this than the primary nitrosamine… These would be expected to be more stable, and there are plenty of examples of carcinogenic nitrosocarbamates - nothing available in LCDB (https://carcdb.lhasalimited.org/) is as hindered, but everything we have is carc positive (unlike dialkyl nitrosamines, which have a reasonable proportion of negatives), and some are potent at CoC-worthy levels.
You’re correct that a-hydroxylation at the adamantyl group is considered extremely unlikely, however if the boc is cleaved (strong acid is the usual condition set, I believe - so a potential hazard with oral dosing or subsequent synthetic steps) the initial query compound can be re-formed, potentially in close proximity to DNA, which matches the hypothesis for other carbamates (non-enzymatic cleavage of carbamate generating alkylating agent in proximity to DNA). The steric hindrance from the boc protecting group may (I don’t know - depends on the mechanism of the cleavage) reduce the ability for the carbamate to be cleaved with respect to other carbamates, so this should be investigated - can a boc group be cleaved in physiological conditions, if it isn’t sufficiently purged in the synthesis?
An interesting aspect to this specific structure, upon reflection, is the adamantyl nature of the carbocation that can be proposed to occur (either as the hypothetical ultimate alkylating agent, if genotoxic, or as an intermediate on the pathway to spontaneous degradation). SN2 substitution of a diazonium, if formed, is impossible due to the nature of the adamantyl group - you can’t do SN2 through it! SN1 is still a possible mechanism, however the cation cannot gain stability from being planar and receiving hyperconjugation from sp3 bonds on the adjacent carbons… this could make it either too unstable to exist, or just the right amount of instability to be reactive - I’m not sure, but it’s certainly quite different to unstrained tert-butyl groups (which would take an sp2 configuration, whereas this is forced into sp3). There’s definitely literature out there on the reactivity of these, so might be worth further investigation.
this is a nitrosation of memantine  What are you doing/saying? Memantine is a primary amine. Primary amine nitrosation eventually leads to elimination of nitrogen to give an alcohol (freshman Organic Chemistry: 21.10: Nitrosation of Amines - Chemistry LibreTexts
What are you doing/saying? Memantine is a primary amine. Primary amine nitrosation eventually leads to elimination of nitrogen to give an alcohol (freshman Organic Chemistry: 21.10: Nitrosation of Amines - Chemistry LibreTexts
This will eventually produce
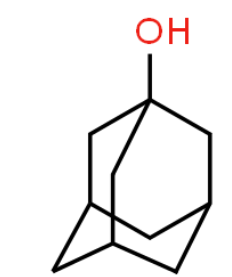
Again, I recommend reading Jerry March, Carey and Sandberg or other elementary college chemistry books
Thanks David, good suggestions and I will share with the customer. -Jie