Hello everybody!!!
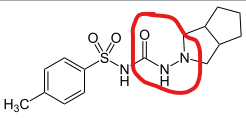
Can anyone help us with a nitrosamine formed during gliclazide synthesis (2-nitroso-octahydrocyclopenta[c]pyrrole).
Link: Octahydro-2-nitrosocyclopenta[c]pyrrole | C7H12N2O - PubChem (nih.gov)
The nitrosamine is formed in initial stages of the synthesis as impurity (GCB or impurity B) and this impurity is possible carryover in the final stages. According our manufacturer, the nitrosamine impurity is controlled in the final drug substance with a limit of “not more than 2ppm” in-line to the Ph. Eur mograph. But our question is this impurity should not be in according to ICH M7(R1)? Other question, there is a suitable acceptable limit adopted for this impurity?
We think that is a highly risk employ this API like raw material in our process due the presence of this impurity in this reported level. Also, the recommended control strategies, in this case, should be control every batch of API an DP (like a routine control) or is possible adopt another strategy?
Many thanks for the help!!!