Screening for Nitrosamines with Thermal Energy Analysis
TEA is able to rapidly identify and analyze N-nitroso compounds without the matrix interference that is common with MS detection. Due to the sensitivity and selectivity for nitroso-containing compounds, TEA has been the preferred method of testing in many industries since the 1960s.
Figure 1: Process showing the breakdown and detection of nitrosamines using gas chromatography-thermal energy analysis.
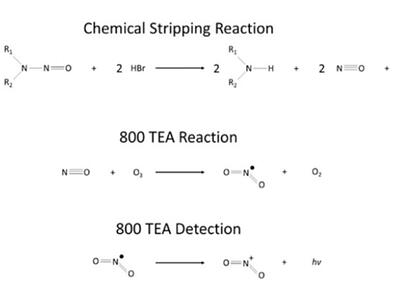
A typical analysis workflow separates compounds using GC before the effluent is directed through a 500°C pyrolyser to initiate a series of reactions (Figure 1) to speciate the nitrosamines. For non-speciated, total nitrosamine analysis, TEA is used after a chemical reaction where the nitrosamines are broken down chemically. The nitric oxide (NO) radical is released due to heat breaking the N-N bond. The gas is passed through a TEA detector. The NO reacts with ozone, which is produced in the unit, to form an electronically excited NO2 molecule. Once this is relaxed, it releases a photon above 600 nm. The NO2 rapidly decays and emits near infrared light that is then detected by a sensitive photomultiplier.
TEA uses selective thermal cleavage of the N-NO bond and the detection of the liberated NO radical reacting with ozone to generate a chemiluminescence signal. TEA systems have a nitrogen chemiluminescence sensitivity of less that 2pgN/second. In addition, TEA detectors can alternate between nitrogen and nitroso/nitro modes, with the latter eliminating nitrogen compound interference.1
An added advantage to TEA is the selection of temperature settings within the pyrolyser to break specific bonds. Bond breakage is largely limited to N-N bonds at 500°C enabling TEA to excel at identifying nitrosamines. Carbon-nitrogen bonds can also be broken by increasing the temperature to 650°C, enabling nitro group identification.3
TEA can also be configured to offer rapid, routine pre-screening of total nitrosamine content in pharmaceutical samples as well as detailed, speciated analysis. Receiving the apparent total nitrosamine content (ATNC), showing both volatile and non-volatile compounds, will enable those compounds below the limit of detection level to be quickly deemed safe. Those compounds with a positive level of ATNC can be further tested to identify the specific nitrosamine compound present.2 The ATNC test allows pharmaceutical manufacturers to meet the requirements of the EMA risk assessment without needing to outsource testing of raw materials or of products at different stages of manufacturing.
Samples for ATNC analysis, rather than undergoing pyrolysis, are injected into refluxing ethyl acetate containing concentrated hydrobromic acid (HBr). This chemical stripping method causes a reaction with the HBr and produces NO, a secondary amine and bromine. The NO is carried toward the TEA by a flow of nitrogen above the headspace of the reaction vessel. A condenser coil prevents the loss of the solvent, and the vapor is cleaned by a secondary cold trap before entering the TEA for analysis.