https://recalls-rappels.canada.ca/en/alert-recall/apo-amitriptyline-10mg-ndma-impurity-0
For reference:
SMILES: c3cc2c(/C(c1c(cccc1)CC2)=C\CCN(C)C)cc3
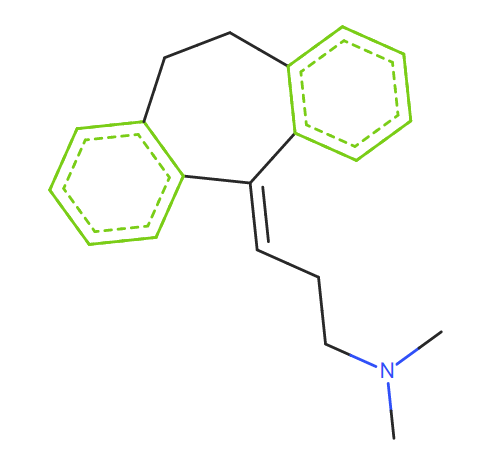
As Amitriptyline is tertiary amine, it should be concerned with not only N-nitrosonortriptyline(AI = 8ng/day) but also NDMA. It’s reasonable but challenging for us.
Amitryptylene, where ever the process involves usage of dimethyl amine in final step, will convert to NDMA during stability . We should consider the case similar to Metformin.
Thank you for sharing the information, @siva. I confirmed the synthetic scheme of amitriptyline with DMA.
I was concerned that some APIs with dimethylamino structure might risk NDMA contamination through direct nitrosation of the tertiary amine. But if they contain DMA as an impurity, NDMA contamination is reasonable!!
N-nitrosonortriptyline AI = 8ng/day which is published in EMA Q&A and there is no any reference in FDA guidance.
As per FDA guidance default limit for any nitrosamine AI = 26.5 ng/day.
Kindly looking for your valuable thoughts about which AI limit for N-nitrosonortriptyline shall be considered for USA submission.
Hi, @Yuvraj.
Thank you for asking me. I’m sorry that I have no experience consulting the AI of unknown nitrosamines with the FDA.
The FDA allows us to use read-across in the guidance. If you suggest the different values from 8ng/day as the AI of N-nitrosonortriptyline, the reason why it is more appropriate than 8ng/ day may be required. I think the default values are applicable for nitrosamines free from read-across and you can suggest 8ng/day to the FDA in this case.
And I’m not sure how the FDA will respond. Does anyone have any additions?
@Yosukemino
Thanks for valuable input and guidance.
Health Canada recalled amitriptyrine due to NNORT contamination. The interim limit is 2.5 ppm according to HC. If MDD is 300mg/ day, it is equivalent to 750ng/ day.
https://recalls-rappels.canada.ca/en/alert-recall/elavil-25-mg-nnort-impurity
It seems that Amitriptyline continues to be recalled due to nitrosamine contamination.
APO-AMITRIPTYLINE Tablets: NNORT impurity
https://recalls-rappels.canada.ca/en/alert-recall/apo-amitriptyline-tablets-nnort-impurity
PMS-AMITRIPTYLINE 10 mg, 25 mg, and 50 mg: NDMA and NNORT impurity
https://recalls-rappels.canada.ca/en/alert-recall/pms-amitriptyline-10-mg-25-mg-and-50-mg-ndma-and-nnort-impurity
AMITRIPTYLINE 10 & 50mg: NDMA and NNORT impurity
https://recalls-rappels.canada.ca/en/alert-recall/amitriptyline-10-50mg-ndma-and-nnort-impurity
I see now interim limit of 2.5 ppm for NNORT removed by HC!.
And also HC used cumulative limit terminology for both NDMA and NNORT- How much it could be and derived on what basis interim or published AI?
Hi, @Pradpharma
I can’t find the removal of interim limit of 2.5 ppm. And some lots were withdrawn due to the cumulative limit over. While interim limit is set for NNORT, it’s difficult to meet the cumulative limit that is lower than single AI. I am interested in the HC’s policy.
I wish to know, NDMA is considered as most potent nitrosamine impurity. Still, AI for n-nitroso nortriptyline is less than NDMA, even though molecule is bigger than NDMA.
Good Question. Unfortunately, N-nitroso nortriptyline has an AI of 8 ng/day which was designated as more potent than NDMA.
This could be due to simple di-alkyl like nitrosamine structural feature.
https://www.mhlw.go.jp/stf/newpage_33904.html
Just to make you aware:
The issue of N-nitroso nortriptyline was evaluated by PMDA-Japan and a good discussion happened on it, Where they considered NDMA as read-across to N-nitroso nortriptyline temporarily just to not cause any adverse impact on patients who rely on nortriptyline because sudden drug shortage may cause withdrawal symptoms to patients who were using for long time.
@Yosukemino - Any further update or progress in PMDA on this?
I want to add that the AI of N-nitroso nortriptyline is derived from the AI of NMPEA through the read-across method. And NNK is recommended as a surrogate for N-nitroso nortriptyline in a great article, Nitrosamine Saga. I agree.
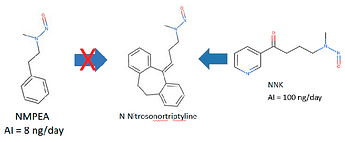
I also want to focus on the decision of PMDA is not for universal AI but for temporary AI.
No further discussion is available, unfortunately.
