Hi
Happy New Year to all
Requesting guidance in the calculation of allowable limit in drug product with two API’s
Eg.
Drug product AB Tablets (MDD as per package Insert for AB is A-20 mg and B 25 mg)
A (First Active Pharmaceutical Ingredient) has MDD 20 mg
B (Second Active Pharmaceutical Ingredient) has MDD 25 mg
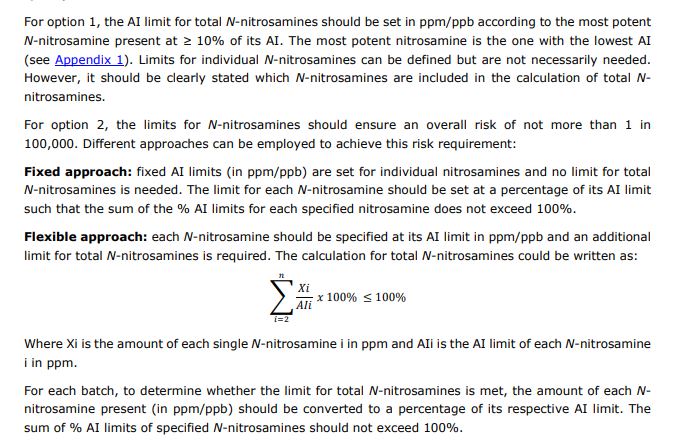
As per USFDA guidance on NDSRI dated Aug 2023 and the list with AI published on Dec 2023 the Recommended AI limit for both API’s [A & B] are 1500 ng/day
The concern is calculation of allowable limit for drug product
1 Like
Thank you so much for the prompt response
We understand the guidance referred is for Nitrosamines only
We are seeking guidance on the NDSRI allowable limit calculation with two API’s in ANDA
This is applicable to NDSRIs.
See also this topic:
Limit for API having two NDSRI - Limits of Nitrosamines - Nitrosamines Exchange (usp.org)
2 Likes