If an API contain Amide group, should it be treated as a potential treat to forming nitrosmaine.
When dealing with amides, one should always keep in mind the possibility of hydrolysis. And whether to treat the amide as a threat, depends on the number of carbon atoms connected to the nitrogen in the amide.
If it’s just one, then the only possible hydrolysis product containing nitrogen would be ammonia, which is neither nitrosable nor a nitrosating agent.
If it’s two, then a possible hydrolysis product is a primary amine. These may undergo nitrosation under favorable conditions, giving primary nitrosamines. But these nitrosamines however are not very stable, and decompose into N2 and the remaining part forms an alcohol.
The problem lies with amides with three carbon atoms connected to the nitrogen. A possible hydrolysis product is a secondary amine, which may undergo nitrosation under favorable conditions, giving secondary nitrosamine. These are stable enough to remain in the API, product and possibly become ingested by the patient.
As (tertiary) amides are fairly stable structures due to resonance (unless in case of specific substitution activating for hydrolysis (e.g. Szostak twisted amides) and reducing amidicity), it is typically more common that contamination with secondary amines is the problem (this depends on the synthesis route applied to the amide applied) or cross-contamination of nitrosamines maintained in the secondary amine starting material. Degradation of amide related root causes are not anticipated to play prior to the end of shelf life typically.
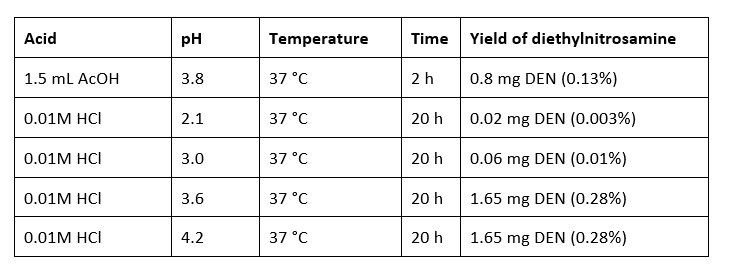
In guidance EMA has also illustrated the limited reactivity of amides with the literature example of nikethamide: reactions based on 1.0 g nikethamide and 1.7 g nitrite:
Data originating from:
LIJINSKY, W., CONRAD, E., & VAN DE BOGART, R. (1972). Carcinogenic Nitrosamines formed by Drug/Nitrite Interactions. Nature (London) , 239 (5368), 165–167. Carcinogenic Nitrosamines formed by Drug/Nitrite Interactions | Nature
See also Table 3 of the EMA report “Lessons learnt from presence of N-nitrosamine impurities in sartan medicines” https://www.ema.europa.eu/en/documents/report/lessons-learnt-presence-n-nitrosamine-impurities-sartan-medicines_en.pdf
So an API-related root cause is not the first thing that comes to mind, considering the low conversions for amides, especially with an identifiable secondary amine starting material (which is not always the case for more complex (cyclic) structures).
I want to add to the link to my post(Strategy to Assess and Control Nitrosamine Formation in API during Storage: A Sitagliptin Case Study). As it may be rare case, residual secondary amine(LT 100ppb) in Sitagliptin Hydrochloride Monohydrate caused nitrosamine formation in storage due to NOx gass in the air.
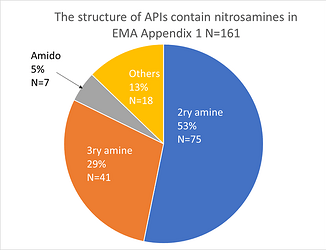
Besides, I classified 161 Nitrosamines in EMA Appendix 1 by structure, 5% was from Tertiary amides.
Nitrosamines with CPCA cat.1 and 2 derived from amides may be concerning in some cases.
Thank you for the valuable input, is there any published article which i can use as reference for my report for the number of nitrogen attached to the carbon and possibility of forming nitrosamine.
does this holds true for secondary amide as well.