Can Ezetimibe be nitrosated?
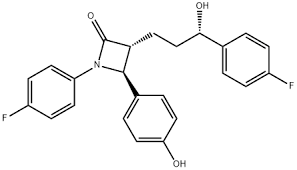
Can Ezetimibe be nitrosated?

Dear PPP,
Ezetimibe is an aromatic tertiary amide. This means that its nitrogen is a very weak base and consequently its nitrosation should be almost impossible even in harsh conditions.
thanks
I agree with the previous comment, but I would certainly look for secondary amines as part of the impurity profile of Ezetimibe. A lactam like this is quite tense as a ring and I would not be surprised if it was prone to hydrolysis. If that happens, you would have a secondary amine as a product.
Dear Javier,
indeed the lactam ring is sensitive to open (specially under basic conditions), but the main impurity which is formed is the ezetimibe tetrahydropyran analog, as the carbonyl group reacts with the benzyl hydroxyl group and resulted, via internal cyclization, to pyran-type ring. This is still an aromatic amide, so no risk regarding the formation of secondary amines is presented even if the lactam ring is opening.
Yes, structurally, it being the amide of a secondary amine can be nitrosated. But the possibility is quite low that ezetimibe will undergo facile nitrosation. On the other hand, the open chain amine is an intermediate in the ROS, that is quite prone to forming nitrosamine.
Dear PPP,
When it comes to your question, I have two thoughts:
Best,
Michal