Dear Team,
Regarding the potential for nitrosamine impurities in Clobazam, I wanted to share some initial considerations.
As per EMA Appendix 1, Clobazam is not currently listed as having a known risk for specific nitrosamines based on its structure and common synthetic routes.
However, our initial assessment suggests a potential risk that warrants further investigation:
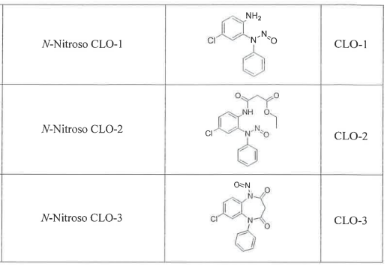
- API Degradation: There’s a possibility that degradation of the Clobazam API could lead to the formation of N-Nitroso Clobazam Impurity E and N-Nitroso Clobazam Impurity F.
- Process Evaluation: Our ongoing process evaluation may also identify potential pathways for other nitrosamine formation.
While EMA Appendix 1 is a valuable starting point, it’s crucial to conduct a thorough risk assessment that considers API degradation pathways and our specific manufacturing process to definitively rule out or mitigate any potential nitrosamine risks.
Let’s schedule a meeting to discuss this further and outline the necessary steps for a comprehensive risk assessment, including potential testing strategies if deemed necessary.