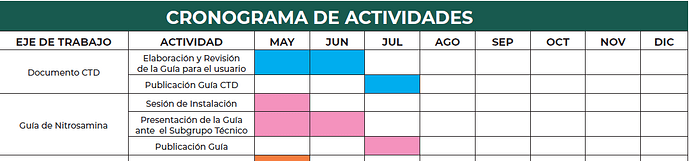
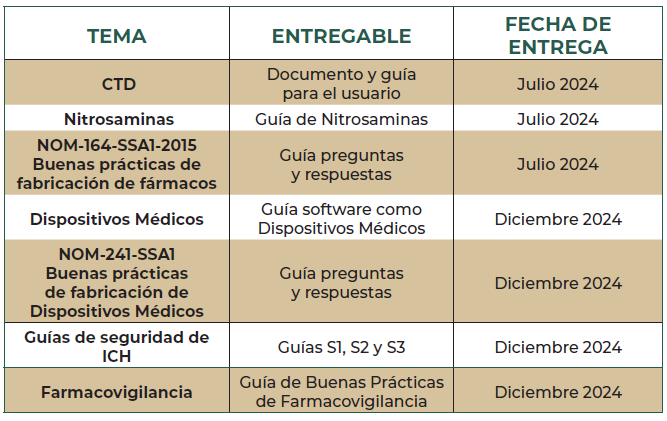
Last week, we learned that the Mexico Regulatory Agency (COFEPRIS) will publish the Nitrosamine Guideline. Industry has highly anticipated the publication since the current call to test Nitrosamines has been conducted via the compendial requirement by the Mexican Pharmacopeia (FEUM).
COFEPRIS Consejo Cientifico.pdf (256.5 KB)
3 Likes
thanks for the heads up. Really useful.
1 Like
Prior to the CPCA Rev. 16 update from EMA, the guideline was expected to be issued mid 2023. Now, it seems to be finally ready for publishing.
Would be really interesting to see how the framework and scope is being set up. Ideally, should be based on EMA/FDA guideline as starting point and avoiding testing “general” nitrosamines without a risk assesment indicating the need to.
I also wonder how small to mid sized companies in Mexico will handle the topic. In the past, by personal experience we saw in some cases the “if there is no guideline in my country, we will not consider the evaluation”, as there was resource constrains + other topics behind, but now with an official document, things will for sure change.
2 Likes
HI Team,
We cannot locate Appendix-1 & Appendix-2 in the english version of COFEPRIS guidance. Will somebody help to locate it?
Thanks in advance
The guide has been published…