Thank you so much for this great tool. This is super easy to use and very handy. Thanks for sharing this with the community, much appreciated!
@Yosukemino
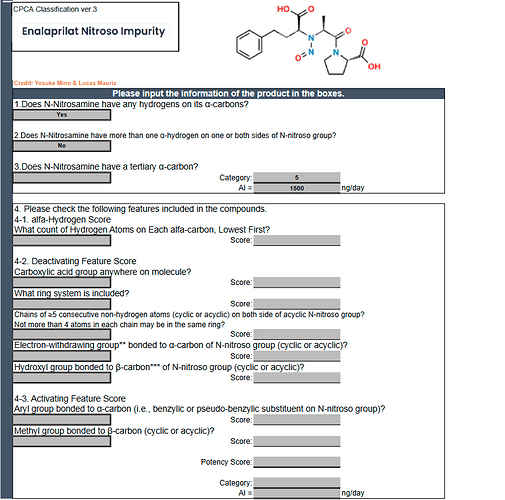
Thanks for responding the query. But under section 4.1 alfa - carbon score, the value added is 1 and the overall category comes to 4. Is the calculation done is correct (excel attached in earlier communication).
@Yosukemino
Thank you for clarifying the query.
Update version 4.2
-including FDA references
CPCA classification spreadsheet v4_2 -Nitrosamine Exchange.xlsx (227.8 KB)
It’s incredible; many thanks Sir, for sharing Excel.
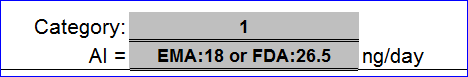
Thanks for the Excel, Can some body help to calculate the AI for N-Nitrodiethanolamine (NDELA) as per US FDA recent guidelines which was published in august 2023
Health Canada > AI of 1900 ng/day in their April update guidance
Lhasa Carcinogenic Database > Gold TD50 3.17 mg/kg/day = 3170 ng/day
Please confirm…
Dear @Debendra, your question seemed a bit unclear to me, but based on the available information, I believe the answer you are seeking can be found in the FDA guidance:
“Generally, FDA has communicated recommended AI limits directly
to an applicant or manufacturer or through an FDA guidance. FDA
may continue to do so, including in connection with this guidance. When FDA communicates a recommended AI limit based on compound-specific assessments or read-across analysis from a surrogate, manufacturers and applicants should apply that recommended AI limit rather than using the predicted carcinogenic potency categorization approach in this guidance to identify a recommended AI limit.”
As written by @Naiffer_Host , we have for NDELA, the value of 1900 ng/day, a limit based on the most sensitive TD50 value derived from the most robust TD50 study available in the CPDB database.
Hello experts/SMEs,
I really appreciate you shared the powerful spreadsheet.
However, should the NDSRI be identified first before proceeding to calculate AI limit?
I have a question for
**how to identify the potential NDSRI in drug product? if no nitrosating agent was used in the formulating process. **
That would be great helpful if anyone can share some opinions/rationales to me.
Please advise.
Thank you!
Hello Dinkar,
USFDA recently released the guidance on “Recommended Acceptable Intake Limits for Nitrosamine Drug Substance Related Impurities (NDSRIs)” in which CPCA approach is defined.
Thank you,
S. Uday
@arim416 Ryan, that’s a great question… I suggest navigating the different categories we have in the community or posting a new question. This thread is dedicated to the CPCA Calculator tool. Feel free to reach out to me via messaging if you need assistance.
Hi Ryan Lee,
Many cases Formulation not using chemicals which contains nitrosating agent / source as well as Amine (2 and 3 amine). You can ask your API source for the "Comprehensive Nitrosamine risk assessment which includes below discussion:
The risk evaluation should not only address risks related to the manufacturing process but also those deriving from the introduction of materials used in the manufacturing process and other potential sources of contamination (e.g. starting materials, reagents, solvents, recovery of materials, equipment, degradation, etc.). Any risk concerning formation and carry-over of nitrosamines should be addressed taking into account the EMA Q&A document and in line with EMA recommendations after procedure under Article 5(3) of Regulation EC (No) 726/2004 and USFDA recommendations.
For Formulation you can consider or assess the risk from Packaging i.e Blister material as a potential source, wherein nitrocellulose primer in a lidding foil was identified as a risk factor. Nitrocellulose acts as a nitrosating agent for secondary amines, present in printing inks, forming N-nitrosamines in lidding foil
Regards,
Dr. Dinkar
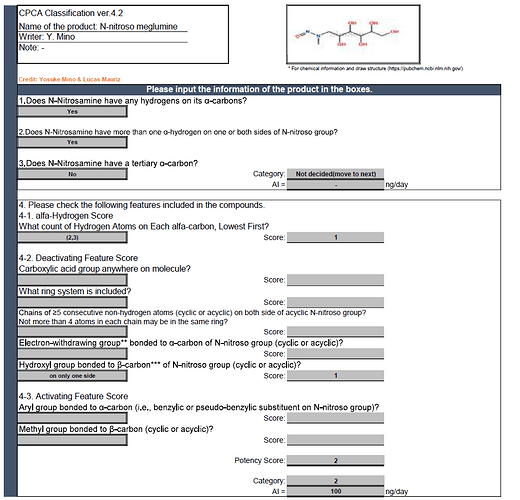
Fantastic job done by @Yosukemino and @lucas10mauriz i need one drought in N-nitroso meglumine please help me is it correct or not?
CPCA classification spreadsheet v4_2 -Nitrosamine Exchange.pdf (72.5 KB)
Hi, @Madhukar
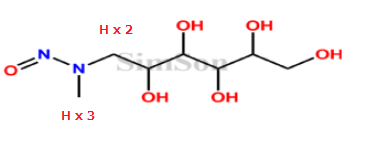
I classified the N-nitroso meglumine as Category 2. Please check the following;
α-carbon is (2,3).
And OH bonds to β-carbon at only one side.
The others are not included in this NDSRI and potency score is 2.
Best regards,
Yosuke
Thanks @Yosukemino for giving reply…
i have a doubt about alpha hydrogens mr @Yosukemino how we calculate for n-nitroso meglumine u shared
2H and 3H are on the α-carbon.
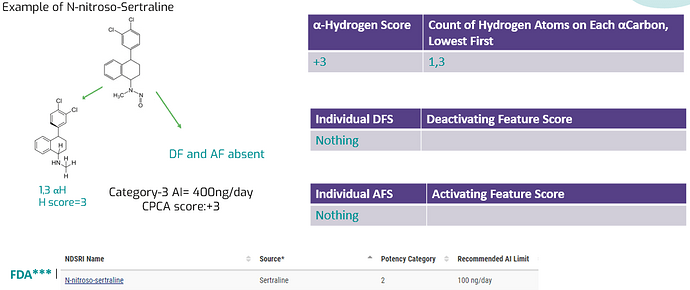
Dear Yosukemino Sir,
Please explain the CPCA Score for N-nitroso sertraline as FDA has kept in potency category 2 (100ng/day) and as Chemical Str. mentioned below.
Additionally, Please explain the cycline case such as (N-nitroso-desmethyl-demeclocycline ,N-nitroso-desmethyl-doxycycline and N-nitroso-desmethyl-tetracycline)
Dear @sandeep0315, I’d like to kindly request your attention to the evaluation of the “activating feature score” category.
Within N -Nitroso -Sertraline, there exists an aryl group bonded to the alpha carbon (score = -1).
Consequently, the cumulative value equates to 3 - 1 = 2 (CPCA 2, AI = 100 ng/day).
Please, what specific query do you have regarding the cyclines? This information will enable my colleague @Yosukemino and me to provide a more targeted response.