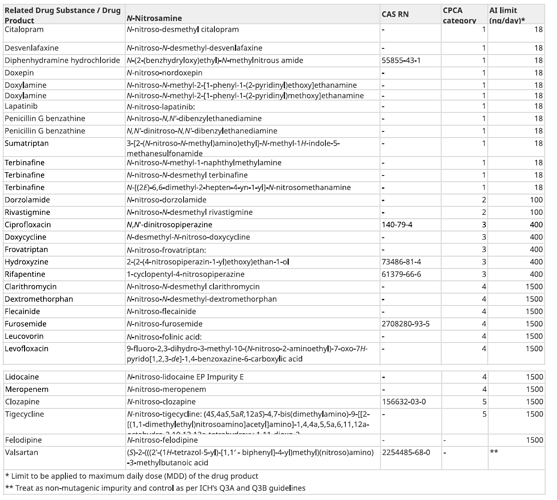
The updates include two significant approaches recently finalized by the Nitrosamines International Technical Working Group (NITWG) that will assist industry and regulators in setting Acceptable Intake (AI) limits for nitrosamines in drug products, namely the Enhanced Ames Test (EAT) conditions and the Carcinogenic Potency Categorization Approach (CPCA). The updates to the guidance document include the addition of AI limits for several nitrosamines that are considered acceptable to Health Canada. The application of the CPCA has revised already established AIs for various nitrosamines, these include N -nitroso-dabigatran, N -nitroso-tamsulosin, N -nitroso-rasagiline and 1-methyl-4-nitrosopiperazine (MNP).
Over to the FDA now???
Thanks Sir for Sharing the news and Guidance, Its is very useful for Pharma Industry.
Now waiting for other Agencies view.
Thanks and Regards,
Dr. Dinkar Gagare
thank you so much!!! ![]()
From what I have seen, in the IA tables there are differences with those of the EMA. They include, for example: the 3 NNO of Terbinafine that are possible and one more than EMA for Levofloxacin…
Now let’s see what FDA publishes… What nerves! ![]()
Thanks for the info.
I have seen some new nitrosamines also. With some interesting insights, for example N-nitroso-desmethyl clarythromycin is there with a limit of 1500 ng/day. It seems that for more than one case a negative TGR will be needed to derisk the topic, considering the Azithromycin ones were derisked with a -ive TGR.
Note apart is that Health Canada I understand is internally (same policies related to NAs) pretty much aligned to EMA, but the FDA is a different case. One of the reasons FDA do not publish new update of their guideline since years ago.
I compared the results of CPCA between EMA and Health Canada. And 32 nitrosamines in the list of HC look not included in that of EMA. HC works enthusiastically to increase the number of nitrosamines in its AI list.
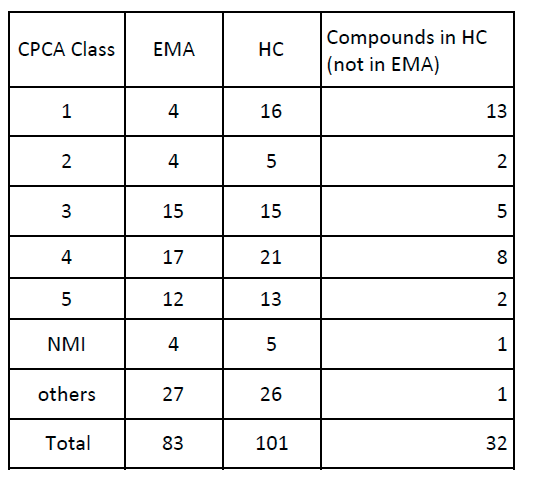
Thanks Yosukemino
Also what that means is that at least in Canada a lot of NDSRIs has been detected, and even ones coming from tertiary amines.
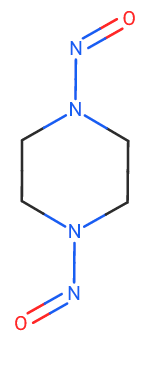
I want to focus on N,N’-dinitrosopiperazine. CPCA is applied to the dinitroso compound. And I wonder whether 2.0 microgram/day is not enough?
@Yosukemino, an analysis of the compiled data from both regulatory agencies reveals a significant finding. Notably, the majority of NDSRIs, comprising approximately 20% of the total, are classified as class 4, indicating a daily intake level of 1500 ng.
We tried to synthesize nitroso impurity of Doxycyline and Felodipine, but not forming related nitroso impurity. Health Canada has published limit for these nitrosmaine. I am confused, how to handle the issue?
Felodipine in particular is an interesting inclusion, given that the “it can’t be formed” argument has as far as I am aware pretty much been accepted for other dipines… (they aromatise under nitrosating conditions)
Would following the approach proposed by Ashworth et al, of trying a set of standard conditions and arguing an impossibility of formation if those don’t work, be a good approach here?
In the AI list on the health Canada page (appendix 2) there are several compounds showing ** in the last column. Meaning: “**Treat as non-mutagenic impurity and control as per ICH’s Q3A and Q3B guidelines”.
Anyone aware if the reasoning behind this approach for these compounds is provided somewhere?
As Health Canada is pretty much aligned with EMA, I could infer it is based on what EMA says, that a negative Transgenic rodent mutation assay (TGRA) was obtained.
Same here we tried to synthesize [N-nitroso-desmethyl-doxycycline] but it is not forming. The same impurity is listed in HC and FDA guidance. Any lead is appreciated.
N-nitrosodesmethyl doxycycline is a metabolite of the drug susbstance. Therefore, possible to be formed, but possible to be formed under common nitrosating conditions? I think in this case, what is recommended is to go to the Health Authority and indicate the molecule cant be sinthetized under meaninful nitrosating conditons related to a drug product or substance manufacturing.
With that maybe the case could be dismissed and EMA for example actually put that option in the table.
Thank You. Appreciate your feedback.