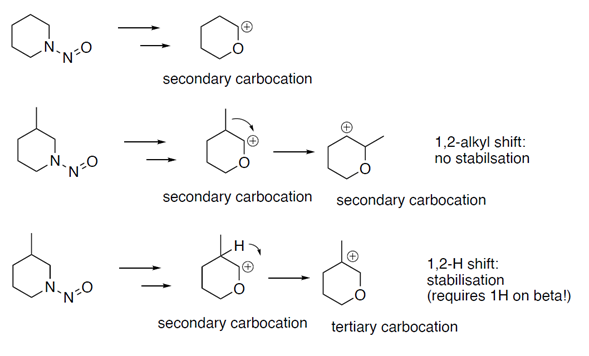
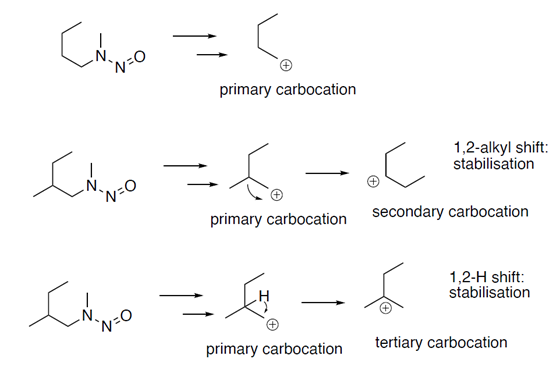
One of the NITWG identified activating features in the CPCA approach (Table 4 of Annex 2 of EMA Q&A Rev. 16) is the beta-methyl activating feature (methyl group bound to beta carbon, cyclic or acyclic):
![]()
The NS OEG has clarified:
The beta-methyl has to do with theoretic motivations of carbocation stabilization, just a chemical-mechanistic reason (theoretical). A more stable carbocation is more likely to form.
(cf. 1,2-methyl shifts and 1,2-hydride shifts)
What do you think about such a theoretic correction factor? Have you seen examples (cyclic or acyclic) where data seemingly verified the activation hypothesis?
Note: shouldn’t this activating feature be disregarded then when on the alpha carbon next to the beta carbon with the methyl substituent there is substitution (no alpha CH2)? For example, application of the -1 score on N-nitroso 3-methylpiperidine but not on N-nitroso 2,3-dimethylpiperidine? If you can’t form alpha to the beta a carbocation in the first place, then you also can’t stabilize it by rearranging to a tertiary carbocation.
Note: the methyl group bounded on the beta carbon should also be a mono substitution then, remaining hydrogen on beta required depending on carbocation stabilization mechanism?
Note: A more alkyl substituted carbocation is more stable due to the electron donating features of the alkyl group stabilizing the positive charge (primary < secondary < tertiary carbocation). An alpha carbocation can only be stabilized by alkyl on beta after rearrangements/shifts in the structure. The more bulkier the alkyl group the less likely the shift of the alkyl to stabilize (1,2-methylshift) and the less likely sterics on beta allowed an alpha carbocation to get formed in the first place. The formed primary carbocation (or secondary in case of cyclic nitrosamine) on the alpha position can be stabilized by rearranging it (hydrogen rearrangement, hydride shift) to a more stable tertiary carbocation on beta. Alternative a 1,2-methylshift (from beta to alpha) would stabilize the alpha carbocation by going from a primary to a secondary carbocation (acyclic nitrosamines), for secondary to secondary carbocation (in case of cyclic nitrosamines) no stabilization rationale.
Note: in Dobo 2022 an equivalent TD50 for 4-methyl-1-nitrosopiperidine and 3-methyl-1-nitrosopiperidine is evaluated from Lijinsky and Taylor studies, thus not allowing verification of the theory. Also the small difference in TD50 data summarized in Dobo 2022 of 1-methyl-4-nitrosopiperazine and 1,2,6-trimethyl-4-nitrosopiperazine does not allow verifying the EMA model. Dobo 2022 also compared the lower TD50 data on 4-nitrosomorpholine to 2,6-dimethyl-4-nitrosomorpholine, which is not necessarily originating from the beta methyl effect.
(Dobo 2022 = Krista L. Dobo, Michelle O. Kenyon, Olivier Dirat, Maria Engel, Andrew Fleetwood, Matthew Martin, Susan Mattano, Alyssa Musso, James Christopher McWilliams, Alexandros Papanikolaou, Patricia Parris, Jessica Whritenour, Shu Yu, and Amit S. Kalgutkar. Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N‑Nitrosamine Impurities. Chem. Res. Toxicol. 2022, 35, 475−489)