@Yosukemino great agenda for the workshop. Please let us know if there is an opportunity for any member of the community to participate or contribute. Also, let me know if you need additional resources about Nitrosamine Exchange.
Thank you, @Naiffer_Host. I will introduce the usefulness of this community in my presentation.
@Yosukemino
If this workshop is online also, please share the link in advance.
Thanks
Nilesh
こんにちわ、日本の皆様
AAPS-Ask AnythingのQ&AをDeepL翻訳で日本語訳したものを作成しましたので、よろしければご確認ください。
AAPS QA with translation.pdf (410.1 KB)
これまで日本では、ニトロソアミンのリスク評価に関する化学的な議論の場を持つ機会が滅多にありませんでした。しかしながら、今後は学会やセミナー、またはこのスレッドを含む様々な機会を通じて、少しでも多くの皆様の疑問を解消できることを期待しております。是非とも一緒に勉強しましょう。
オリジナルはこちらです。
業界側の相談窓口は、相談の取りまとめをするための窓口で、行政宛てに一斉に問い合わせを送られても対応できないからといった理由らしいです。詳しい続報を待ちたいですね。
こんにちわ、日本の皆様
10/14の技術教育シンポジウムはいかがでしたか?
今話題のt-AIに関するEMAのDr.Breamや@AndyT のコメントが聞けて、非常に素晴らしいものだったと思います。
主催者の檜山先生によると、Q&Aも含めて文書での情報提供を考えているそうです。
個人的に気になったことを簡単に記載します。
- ニトロソアミンの国内通知等の動向に関して (福地先生@PMDA)
福地先生が昨年10月に公布された国内通知の内容を紹介。
・リスク報告が4/30までなのに対して、Q&Aがなかなか公開されないことについて先生も心配している。
・ニトロソアミン規制に関して、日本固有の事情がある(以下、例)
- M7はニトロソアミン管理の基盤の一つといえるが、国内では後発医薬品がM7の対象でない
- USP、EPと違い、JPではニトロソアミンの試験法を公定書に記載していない
幸いにも海外が進んでいるので、国際協力を進めてうまくキャッチアップしたいとのこと。
Q.国内通知は内容が少ないが、規制が緩いということか?
A.EMA/ FDAのガイダンスを参照することで、海外と同等のものとなっている認識。きちんと国際調和させる。
Q.国内通知に「がん原性試験データを利用出来ない場合は構造活性相関又は遺伝毒性試験に基づき限度値を設定する」とあるが、この遺伝毒性試験はどの試験か?
A.特にどの試験とは限定していない、ケースバイケースで補助的に用いて妥当性を説明する
- 医薬品におけるニトロソアミン類の分析法 -国立衛研の取り組み― (内山先生@NIHS)
内山先生が、NIHSでのニトロソアミン分析の事例について紹介
・バルサルタンはMSがなくても分析できるようにHPLC-UV法を開発。
・同じ原薬製造所(@中国)で製造されたバルサルタンでも、製剤によってNDMAが検出されたものとされなかったものがあった。製造委託元によって原薬の製造方法が異なっていると考えられる。
・ラニチジンは、保管条件によって分解してNDMAが生成。
・メトホルミンは、原薬不純物のDMAが包装資材のニトロセルロースと反応するのと包装資材とは無関係の2パターンあった。
・NDMAは酸性での分解が懸念されていたが、酸性条件下の分析は問題なし
・SPE抽出を用いて高感度分析が達成可能であった
(続きます)
@Yosukemino, 続きをどうぞ 


(続き)
- 原薬製造におけるニトロソアミン類の管理 -サクラミル原薬を事例に- (長遠先生@Fujifilm)
長遠先生がICH Q9に従ったリスク評価法を用いてニトロソアミン混入のリスク評価をする例を紹介
・リスク特定として、EMA Q&AのRoot causeをFishborn diagram → check listに変換し、供給業者に回答を依頼する。
・得られた結果を製造工程のステップごとにFlow chartにまとめる。
・Flow chartをもとに、FMEAを用いてSeverity(品質または患者への影響の大きさ)、Detectability(検出または管理可能かどうか)、Probability(混入が起こりうる可能性)を尺度にスコア化し、ニトロソアミン混入リスクが許容できるかどうかを判定する。
・仮想の医薬品としてCTDモックが作成されているサクラミルを用いて、FMEAの架空のデモンストレーションを実施。ワーストケースを想定した評価ではニトロソアミンの生成・残留リスクが否定できなかったが、該当二級アミンのWHO NAPテストでニトロソ化反応がほとんど進行しないこと(定量限界である0.05%未満)を以て、推定含有量(計算値)がAIの10%を下回ったことから「混入リスクがないため管理不要」と結論付けた。
- 沢井製薬の後発医薬品開発における製剤中ニトロソアミン類の管理戦略(山本先生@Sawai)
山本先生がSawaiにおける開発段階のニトロソアミン混入のリスク評価の考え方を紹介
・ニトロソアミンリスクに関して原薬を10種類に分類(1. ニトロソアミン混入が既知の原薬、2. 原薬にNO2と既知アミンがある、3. テトラゾール環を持つ、4. 原薬製造工程で既知アミン源使用、5. 原薬が既知アミン、6.原薬にNO2と未知アミンがある、7. 原薬が未知二級アミン、8.未知三級アミン、9. その他のリスク、10. リスク無し) その結果、約95%でリスクありとなった(上位:4. 9. 7. 8.)。
・未知ニトロソアミンでは、リードアクロス法によりPractical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N-Nitrosamine Impurities等を参照し、限度値設定する。
・添加剤中の反応性NOx(亜硝酸等のニトロソ化剤)の算出方法として、DMAを添加して密栓し60度で静置し、NDMA量を分析した。本結果を添加剤メーカーへのアンケート結果と合わせて、データベース化した。
・構築した「反応性NOxデータベース」を用いて、ニトロソアミン混入量をシミュレーションした。また、反応性NOxの量が多く、ロット間差の大きい添加剤については、シミュレーションでAIの10%以下になることが予測されても、管理戦略として受け入れ試験で反応性NOx量を測定した事例が紹介された。加速試験でもAIの10%以下を超えなかったことから、本シミュレーションは非常に有効と言える。
(続きます)
- EU experience of nitrosamines impurities: lessons learnt and future perspective (Bream先生@EMA)
Bream先生が今までのニトロソアミン混入事例とEMAの取り組みについて紹介
・バルサルタン-ラニチジン-メトホルミン-リファンピンでのRoot causeを説明
・EMA Q&Aの内容を説明。AIの30%や10%AIでのスキップ試験やOption4といった管理戦略は、Root causeが明確となっている場合に適用されることに注意(分析結果のみでは不適切)。
・Nitrosamine International Technical Working Group(NITWG)がRoot causeの詳細を記述した論文を journal of pharmaceutical sciencesに投稿済み(現時点で未発表)。
- Current Status of N-Nitrosamines – are we approaching the end game? (@AndyTeasdale @AZ)
Teasdale先生が原薬・製剤でのニトロソアミン混入リスクとNDSRIの問題について紹介
・原薬ではニトロソ化剤を製造に使用する例はレアケース。水中の亜硝酸のリスクも、混入量が少ないことから無視できる。ニトロアミンの生成要因は十分に特定されていると言える。
・製剤では添加剤中の亜硝酸によるNDSRI生成に注意。包材に使用されるニトロセルロースにも注意。
・Step1が2021年3月に終了し、Step2に進む必要があったのは5-10%。その他についてはModeling(5APIs/15DPs)、Scavenging/scientific argument(22APIs)、API/Intermediateでの分析(5APIs)、APIでのパージファクター計算(22APIs)、DPでのパージファクター計算(29APIs)、Ames test(33DPs)、添加剤での亜硝酸量分析(2DPs)でMitigationを説明可能であった(カッコ内は適用されたAPIまたはDPの数)
・現在の課題:Ames試験(Ames試験陰性のみではニトロソアミンの変異原性が否定できないことについての整理、これは合理的でないと考えられるが、合意までおそらくまだ1~2年程度かかる)、AI設定、分析上の課題(AI:18n/dayは達成が難しい場合がある)
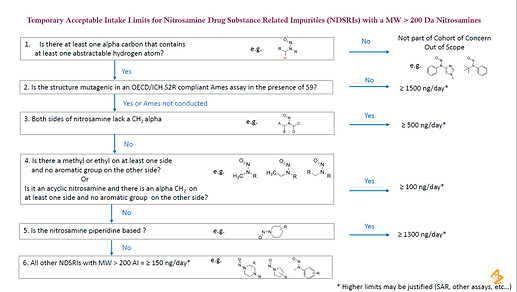
・新しいAI設定のワークフローの提案があった: 1. α-水素の有無?(Noでscope外)2. Ames試験で陽性?(Noで1.5ug/day以上)3.α-炭素は両方ともCH2ではない?(Yesで500ng/day以上) 4.α-炭素の一方がメチルまたはエチルで、反対側が芳香環ではない 等(Yesで100ng/day以上)5. ニトロソアミンがピぺリジン環ベース?(Yesで1300ng/day以上)6.分子量200より大で150ng/day以上
これは,多くの知見が含まれた非常に実践的なものであるが,未だ合意されていな課題がいくつも存在することから,実用化まではまだまだ時間が掛かるものと考える。
- US experience with nitrosamines in pharmaceuticals: risks and mitigation strategies (Keire先生@FDA)
Keire先生が今までのニトロソアミン混入事例と分析上の課題について紹介
・抗酸化剤やpH調整のMitigation方法の有効性は製品によるところが大きかった。やはり実際の検証が必要と思われる。
(続きます)
(続き)
以下、Q&Aから。
Q.アンケートを送っても協力が得られない場合はどうすればいいか?
A.活用できる情報を探すか、自分で測定する。
Q.包装資材のリスクアセスメントはどうすればいいか?
A1.包装資材のRoot causeはニトロセルロース由来であり、ワーストケースでも混入リスクは低い。(メトホルミンの様に)大量に摂取する製剤でしか問題にならないと考える。Mitigation方法としてはニトロセルロースを他成分に変更することだが、今本当に変える必要があるかをよく検討することが重要。これから申請する製品であれば変更すればよい。またベンチレーションにより包装時の排気を十分に行うことも有効と考える。
A2.ニトロセルロースによるニトロソ化反応の詳細については正直black boxである。メトホルミンの事例ではAPI中の残留アミンにより錠剤表面でニトロソ化反応が起こったと考えられる。シーリングプロセスの見直し等が考えられるが、やはり大きなリスクではないので、変更が現実的かどうかを考慮してケースバイケースで対応する。
Q.製剤で使用する水のリスクアセスメントはどうする?
A1.APIでのリスクは低い。それに対して製剤製造中の造粒時等に使用する水によるニトロソアミンの混入リスクについては、詳細があまり知られていない。これから情報が出てくると思うが、少し時間がかかると思う。
Q.リスクアセスメントはどこまで実施するのか?分析は必須なのか?
A1.ケースバイケースとしかいえないが、亜硝酸、(低級)二級アミン等の物性はよく知られているので、必ずしも分析は必須ではないと考える。
A2.国内ではまだまだ知見が十分ではないが、必須ではないと考える。逆に分析結果があればいいというものでもない。パージファクターを用いた検討も有用と言える。
A3.医薬品製造にどうしてもアミン源は存在する。EUでは60万の医薬品が市場に存在することから、それらの全てについて試験をすることは不可能。APIでは科学的考察、パージファクター等を有効に使う。試験ではなくて「品質を確認すること」が大事。
A4.GMPはGMPとして分けて考える。コンタミ等のGMPの問題はGMPできちんと対応する。二級アミンを用いている場合や、三級アミン中に不純物として二級アミンが存在する場合等で、ニトロソアミンの混入が予測できるものについては、適切に対応する。
Q.未知ニトロソアミン(NDSRI)は18ng/dayとするか、リードアクロスをするか? シタグリプチンではEMAとFDAで限度値が異なっている(37ng/dayと246.7 ng/day)。
A1.たとえシンプルな構造であっても、多くのニトロソアミンでは頑健な安全性データがないのが現状。現時点ではAI算出方法について合意に至るのが難しい。まだまだ1~3年程度かかる。とりあえずtemporary AIで1年間は時間が稼げる。
A2.EMAでも他の規制当局と頑張って調整しているが、各市場固有の問題や考え方の違い等があり、globalな調和には時間がかかる。現在EMAでは学術機関に委託して研究を進めており、頑健な安全性データの取得や過度に保守的でないリードアクロス法の構築に取り組んでいる。
A3.α位のプロトンがないものはCohort of Concernでなくていいと考える。構造類似性・代謝メカニズム等を十分に考慮する。
Q.NDSRIについて、国際調和はどうなっているのか?
A1.発がん性データのないニトロソアミンについては、発がん性の強さを数値を用いて定量化できないことが問題。協議はしているがサイエンスが確立されていない。結論が出るのは早くとも2024年の初めくらいにはなるのではないか。
A2.PMDAはリソースが足りていない。M7ではサロゲートが多いが、ニトロソアミンではサロゲートが限られているため、どうしても発がん性ありという前提で進めざるを得ない。またPotencyの強さが議論できないため、現状あるデータを用いてread-acrossすることになってしまう。
Q.FDAで言っているMitigationについて教えてほしい。WHOのNAPテストは必要か?製法を変える、処方を変える等のアプローチがあるが実際のところはどうか?
A1.添加剤中の亜硝酸塩を減らす取り組みは進められており、汎用されているものについては今後1ppm以下にすることが達成できると考えられるが、1ppm以下でも十分とは言えないのが現状。スカベンジャーを入れることが言われているが、それによる製剤全体への影響は不明であり、たとえニトロソアミンが減らせたとしても別のネガティブな影響があるかもしれない。暫定187ng/dayをうまく活用して進めるのがいい。
A2.スカベンジャーはパズルの1ピースのように他の要素と複雑に絡み合っている。そのため実際に行うとなると、pH変える、湿式造粒を止める、処方を変える、データを取得してリバリデーション、実測データ取得等、数多くの段階が存在する。また、その結果、不純物が変わってしまうかもしれない。時間を掛けて議論を続けて、安全性を考慮しながら少しずつMitigation方法の改善を続けていくしかない。
以上です。
後日、運営側から文書として公開されると思いますので、正式版はそちらをお待ちください。
Do you think we could have access to the details of the method that was referenced by Prof. Uchiyama?
Do you think we could get access to the presentations?, it seems like great case studies… Thanks for sharing all the great insights!
Hi, @Naiffer_Host.
We received Dr. Uchiyama’s slides today. I share references for her presentation.
Yes, Dr. Nagato’s presentation is excellent. However, it includes lots of Japanese words. I think it is too difficult to translate into English.
The PDA team posted the report to the “PDA Journal of GMP and Validation in Japan” last time. When the report for this year discloses, I will share the link.
こんにちわ、日本の皆様
10/21の変異原学会の ICH M7/QSARワークショップは、200人以上の参加があり、議論が盛り上がって非常に有意義でした。先生に講演資料の共有も進めて貰っていますので、少々お時間をいただければと思います。
さて、講演内であった大気中のNOxガスの影響による、流動層造粒時のニトロソアミン形成の問題ですが、調べてみたところ以下に関連するスレッドがありました。
こちらは詳細な条件は不明ですが、ニトロソアミンを含まないサルタンAPIが、保管中にニトロソアミンの混入を認めたとのことで、大気中のNOxガスの影響を懸念しており、包装の適切性(LDPE等、透過性のあるものを避ける)について言及しています。DMA、TEA等のアミンの起源については不明です。
流動層造粒時は水を使い、熱もかかることから、特に二級アミンを含む原薬で、かつ塩酸塩等でpHが酸性になる場合は、外気から取り込まれる大量の空気に含まれるNOxガスの影響は、大きなリスクとなる可能性があります。他の医薬品においても同様にリスクとなるのか、更なる調査が必要ですね。
Thank you so much for sharing these valuable information! It could be a great help for all of us who are working for the nitrosamine issues.
I was wondering if you can provide more information on this new strategy to set AI of NDSRIs presented by @AndyTeasdale @AZ
[quote=“Yosukemino, post:31, topic:1626”]
・新しいAI設定のワークフローの提案があった: 1. α-水素の有無?(Noでscope外)2. Ames試験で陽性?(Noで1.5ug/day以上)3.α-炭素は両方ともCH2ではない?(Yesで500ng/day以上) 4.α-炭素の一方がメチルまたはエチルで、反対側が芳香環ではない 等(Yesで100ng/day以上)5. ニトロソアミンがピぺリジン環ベース?(Yesで1300ng/day以上)6.分子量200より大で150ng/day以上
[/quote].
Thank you!
Hi, @heajin.
Thank you for asking. I shared the workflow suggested by @AndyTeasdale. It’s excellent!! I hope it will be officially used in the future.
Hi @Yosukemino
Thank you so much for sharing this slide!
I guess
1500 ng/day comes from TTC 1.5 ug/day
100 ng/day comes from AI of NNK (EMA QnA)
1300 ng/day comes from AI of NPIP (EMA QnA)
I was wondering… where did other two limits, “500 ng/day” for both side of nitrosamine lack a CH2 alpha and “150 ng/day” for all the other NDSRIs with MW > 200 ??
I guess it’s a similar strategy to the article, “Practical and Science-Based Strategy for Establishing Acceptable Intakes for Drug Product N -Nitrosamine Impurities”. The limits do not come from a single compound but from the surrogate such as the lowest one in the group, I think.
@AndyTeasdale, I would appreciate it if you could help us.
@AndyTeasdale was having issue with his logging, but it has been resolved. Thanks