Publication “Current threat of nitrosamines in pharmaceuticals and scientific strategies for risk mitigation” by Tuesuwan et al
Link: https://www.jpharmsci.org/article/S0022-3549(23)00030-8/fulltext
Abstract:
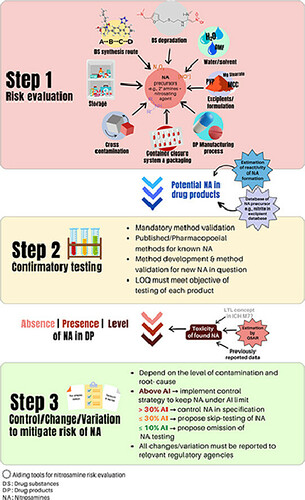
The current global situation of nitrosamine contamination has expanded from angiotensin-II receptor blockers (ARBs) to wide range of medicines as the risk of contamination via the drug substances, formulation, manufacturing process, and packaging is possible for many drug products. The understanding of chemistry, toxicology, and root causes of nitrosamines are mandatory to effectively evaluate and mitigate the risks associated with the contaminated mutagen. Lessons learnt and scientific findings from previously identified root causes are good examples on how to perform effective risk assessments and establish control strategies. Addressing the risk of nitrosamine contamination in pharmaceuticals requires significant knowledge and considerable resources to collect the necessary information for risk evaluation. Examples of the resources required include a reliable laboratory facility, reference material, highly specific and sensitive instrumentation able handle trace levels of contamination, data management, and the most limited resource - time. Therefore, the supporting tools to assist with risk assessment e.g., shared databases for drug and excipients in concern, screening models for the determination of nitrosamine formation potential, and an in silico model to help with toxicity estimation, have proven to be beneficial to tackle the risk and concern of nitrosamine contamination in pharmaceuticals.