Hi everyone,
If Maximum Daily dose for Rosuvastatin is 40mg then for a Nitrosamine with AI=26.5ng/day the limit is 26.5ng per day/40mg = 0.66ppm.
What is the limit of this nitrosamine in the Drug Product?
Is it still 0.66ppm ? or
Since the API is not the only possible source of nitrosamine, the limit should be AI / MDD of Drug Product and not of API MDD?
Meaning 26.5ng per day / 360mg = 0.074ppm (where 360mg is the weight of a tablet of Rosuvastatin 40mg, i.e. the MDD of the Drug Product in weight terms).
what is your view on this?
@Yosukemino @David any guidance on this question? Thx
@e.koustoumpardis @Naiffer_Host
Thank you for asking this good question. While it may confuse you, API MDD is used for calculation.
FDA guidance describes “the conversion of AI limit into ppm varies by product and is calculated based on a drug’s maximum daily dose (MDD) as reflected in the drug label (ppm = AI (ng)/MDD (mg))”.
And MDD is described in labels as follows;
API MDD is used as MDD. This is why 0.66ppm is still applicable for the limit of nitrosamine in the tablet.
In the analytical method of NDMA in Ranitidine Drug Substance and Drug Product published by FDA, you can find the concentration of test solution for the drug product is the same as that for drug substance, 30 mg/mL of API. If you have further questions, please do not hesitate to post them. 
Dear Yosukemino thank you for your answer. The approach you described is followed by the majority of finished drug products manufacturers and although it might be more convinient analytically speaking, it still does not make sence to me since the API is not the sole source of nitrosamines…
Anyway, thanks again.
What also complicates this is if you are talking about more than one N-NO. Guidance around management of multiple N–NOs is complex and not entirely consistent across authorities and their guidance. Recent positive discussions around this topic suggest that guidance may soon be simplified - watch thus space
Dear all.
In my opinion the nitrosamine quantification should be done similarly to other impurities as per ICHQ3B
Yosukemino is right with the MDD because you can consider the tablet as the “carrier” of the API so
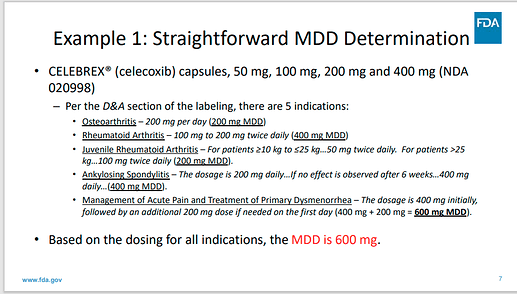
if a physician will prescribe for example 3 tablets per day you have to relate that to the API quantity which is maximum possible. Of course if you have any other excipients subjected to nitrosamine risk you should add them simillarly based on the quantity of that excipient per unit dose correlated with the MDD. I tried to explain also to myself and please correct me if you think I am wrong. The example for Celebrex is quite good. So if only your API is subjected to nitrosamine possible risk you should take in the consideration the maximum quantity of API which might be prescribed as MDD.
Best wishes,
Marcel
@e.koustoumpardis I’ll add my two cents here. The limits have been calculated based on the Maximum daily dose, in your example will be situation 1 the correct calculation. Do not use the tablet weight for this calculation.
The example that @Yosukemino added on the post illustrates that it’s not just the maximum dose manufactured but the maximum dose potentially prescribed by the clinician. In the FDA example is 600mg as MDD
As @AndyTeasdale mentioned all this is considering that you are referring to only one nitrosamine present, if there are more than 1 the situation takes a different direction on the calculation.
Thank you all for your inputs. When more than one Nitrosamine is potentially present things are getting complicated especially if you have to come to terms with an external lab about how strict the LOQs and LODs, depending on guidances interpretation. As Andrew mentioned things have to be simplified.
ICH Q3B - source is the API -MDD from API in calcs
ICH Q3D - source is the (components of) finished product -MDD from finished product in calcs
Nitrosamines - source ??? - MDD???
Marcel described the best approach but how complicated it gets if excipient and/or any other source has to be correlated with MDD each time?
Anyway until everything becomes clearer we should stand up for the decision we make.
Dear all, thank you for the clarification. I guess the difference between Q3B and Q3D comes from the source of impurity, API, and the others. Impurities are defined as degradation products of API in ICH Q3B. And the impurity in API at 0.1% level is considered that in drug products at 0.1% level after formulation if no further degradations. Nitrosamines are treated as the same as other organic impurities in Q3B.
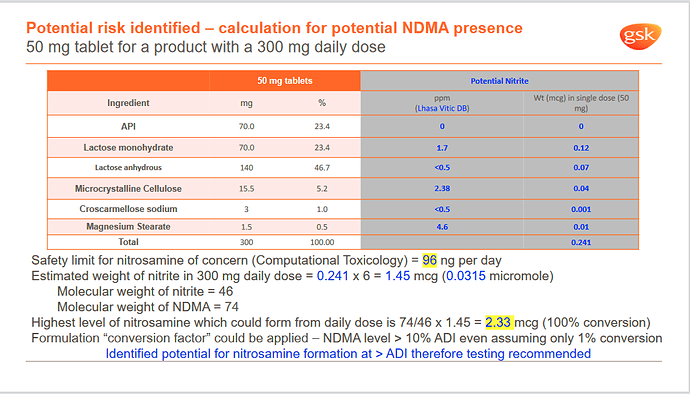
The following is from Dr. Urquhart’s presentation in Lhasa Nitrosamine workshop. It demonstrates the risk assessment of NDMA derived from nitrite in excipients. To calculate the total weight of all possible nitrosamines in drug products first and then translate them into concentration is a good way.
Dear colleagues, below is the answer I took from EMA regarding this issue:
<<Dear Mr Koustoumpardis
Thank you for your question.
Please refer to Q10, page 12 of the same document where it is stated
*The conversion to a specification limit in ppm for a particular medicinal product is calculated by dividing the respective above limit (ng) by the maximum daily dose (mg) of a given product as reflected in the SmPC."
This refers to the amount of drug substance in the finished product and not the total weight of the product including excipients.
Best regards
European Medicines Agency>>
@e.koustoumpardis That settles the question raised in the post, Thanks for sharing the response from EMA.
@Naiffer_Host @e.koustoumpardis
So if I’m understanding it correctly, to calculate the maximum amount (in ppm) of a single nitrosamine, present in the API of a pharmaceutical product, you should divide the AI limit of the perticular nitrosamine (in ng/day) by the Maximum Daily Dose (MDD) of the API present in the pharmaceutical sample?
A theoretical calculation example:
Weight Drug: 500 mg
MDD drug: 50 mg
Weight API in the drug: 100 mg
MDD API: 10 mg
Possible present nitrosamine in the API: NDMA
AI limit NDMA: 96 ng/day
Max amount NDMA present in the API:
96 ng/day / 10 mg = 9,6 ppm
This means that the total amount of NDMA present in the pharmaceutical product may not be higher than 9,6 ppm.
Did I understand this correct?
Hi everyone,
Thanks for the great exchange here, very valuable!
Another question concerning the limit of nitrosamines (not API related!) in drug products; I think it was also mentioned by @sameer here: [Calculation of limit when more than one nitrosamine is identified - #9 by sameer]
How would you determine the MDD to calculate the limit in multiple-API products?
Suggestions:
a) highest MDD of all APIs (e.g. MDD 1 = 10mg, MDD 2 = 20 mg, MDD multiple = 20 mg)
b) the sum of all MDDs (worst case?, e.g. MDD 1 = 10mg, MDD 2 = 20 mg, MDD multiple = 30 mg)
c) any other?
For API-derived nitrosamines, the MDD of the corresponding API would be appropriate; I am just wondering how to proceed with API-non-related nitrosamines.
I would appreciate any feedback
Yes Tom you have understand it correctly according to EMA answer.
However I still have some doubts about MDD (finished product vs API).
An expert during live USP Q&As webinar on 1469 Nitrosamines back in December answered that finished product MDD should be used.
Also in a recent webinar for NDSRIs by European Pharmaceutical Review a Laboratory specialist insisted that acceptable limits should be calculated using finished product MDDs.
Anyway I think that authorities should be more clear in their directions.
Not sure, but I think the individual limit should apply instead of clubbing all the MDDs. This case is similar when we need to take concomitant medicines for multiple diseases. I will share once I have more guidance reference.
Hi everyone,
I just read this and was a bit confused.
When we send a sample for nitrosamines analysis in QC lab, we expect to get results in ppb = ng of nitrosamine per gram of sample.
For example, let say the QC lab result is 100 ng/g of nitrosamine found in a sample of a 1g tablet. This is a factual analysis measurement, so whatever the API content is 10mg, 100mg or 1g in that 1g tablet, and wherever the nitrosamine comes from, the QC lab will give me the same factual 100 ng/g result.
Then if the API content is 100mg per tablet in this example, and the max daily dose is 300mg, it means the patient will take 3 tablets per day: 3g. The patient will be exposed to 300ng of nitrosamine per day in this example.
Totally agree with you. When I read the thread, I also felt confused.
@Yosukemino @Naiffer_Host what do you think?
Thank you for asking, @Alaaelkazak. It’s a common concept in dealing with impurities in drug products. When deciding the criterion of the impurity on DP, we should adjust the limit by API’s MDD. EMA Q&A Q10 described as follows;
The conversion to a specification limit in ppm for a particular medicinal product is calculated by dividing the respective above limit (ng) by the maximum daily dose (mg) of a given product as reflected in the SmPC.
The maximum daily dose is defined in line with the definition of the product strength in the Guideline on the SmPC. Therefore, the limit in ppm should usually be expressed per active moiety (free base, free acid or anhydrous/non-solvated material) for control point in the FP. Exceptions to this are active substances in medicinal products where the strength has traditionally been expressed in the form of a salt or hydrate and active substances present in the formulation as ester or pro-drug.
For a control point in the API only, the limit should be expressed in general per drug substance (i.e. relating to form of salt, hydrate, solvate etc. where relevant).
If you still feel confused, it is helpful to consider the total amount(ng) of nitrosamine contained in DP. The specification (or conversion) itself is not a serious concern.
Thanks dear for your prompt response.
I will give an example to explain my confusion:
Let’s assume that we have a product that its maximum daily dose is 100 mg. The highest strength of the Drug product is 100 mg tablet.
The AI is 37 ng per day.
The limit in ppm should be=AI/MDD= 37/100= 0.37 ppm equivalent to 370 ppb
The problem is: when the nitrosamine content is analyzed in the product (tablet), the results are usually expressed in ng/g of the DP blend/formula.
Let’s assume that we obtained an actual analysis result of 32 ng/g ( equivalent to 32 ppb)
In this case, this results will be 8.6% of the AI which is very good result as below 10% of AI.
But, if we calculated this results with respect to the total tablet weight ( as the analysis already done on the tablet blend); let’s assume the 100-mg-tablet weight is 400 mg:
The nitrosamine in 400 mg= 400*32/1000=12.8 ng
This result is equivalent to 34.59% of the AI, which totally different than the 8.6% ( far higher).
I hope my example is clear enough.