Dear all, thank you for the clarification. I guess the difference between Q3B and Q3D comes from the source of impurity, API, and the others. Impurities are defined as degradation products of API in ICH Q3B. And the impurity in API at 0.1% level is considered that in drug products at 0.1% level after formulation if no further degradations. Nitrosamines are treated as the same as other organic impurities in Q3B.
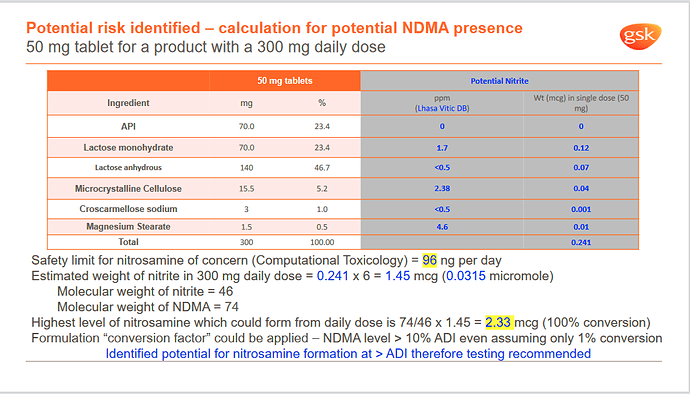
The following is from Dr. Urquhart’s presentation in Lhasa Nitrosamine workshop. It demonstrates the risk assessment of NDMA derived from nitrite in excipients. To calculate the total weight of all possible nitrosamines in drug products first and then translate them into concentration is a good way.