An Ames positive NDSRI is present in levels exceeding the CPCA limit in the drug product; the NDSRI forms during storage/shelf-life; once removed it builds up again. My question is: does anyone have any experience in defining an acceptable intake based on the levels that are technically feasible to control? If yes, how has the control strategy been demonstrated?
thank you
I think that the only (temporary) solution may be the proposal to the authority to apply an LTL limit (se Q22 of the EMA Q&A). In any case the limit should not exceed 1.5 μg/day (apart from few exceptions in Q22).
In any case you should test all the excipient for nitrites and find the one (or the main) excipient which is the root cause.
If you are lucky, the same excipient with a lower content of nitrites may solve the problem, e.g. a cellulose with low nitrite content:
A Low Nitrite Microcrystalline Cellulose
Otherwise you should consider a change of one or more excipient (i.e. a reformulation of the drug product).
You’ve run into the real problem. According to recent guidance, at most you can argue a temporary limit to ensure the drug is not taken off the market. You will not be able to argue for a lower limit based on what is practically achievable.
Solving the problem is a different story. I would consider:
Shelf life changes
Bottling/storage changes primarily to keep the drug dry and/or cold
Low nitrite excipient suppliers
Reformulation with nitrite scavengers
Process changes to reduce moisture and NOx during manufacturing
Kind of a topic. You will need for certain the use or a nucleophilic trap or a nitrosating agent scavenger (like antioxidants). Lets remember that nitrosating reactions the formation rate is from reactant to products and viceversa, you could denitrosate something, but is there is no trap later on, the nitrosamine could be formed again. Something more difficult to solve in a finished porduct manuf. process. Then the other solution is to limit the availability of nitrosating scavengers on all the manufacturing and of course that may end in a big regulatory variation.
Temporary solutions agreeable with EU regulators are in principle not limited to LTL application in the sense of Q&A22 (but the LTL factors often come back in such temporary AIs in EU and US).
Implementation Process_Article 5(3) Nitrosamine (europa.eu)
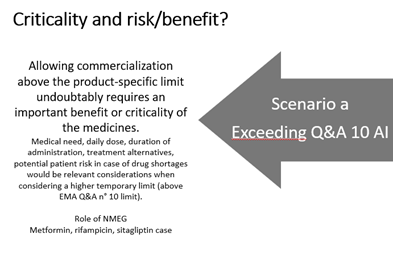
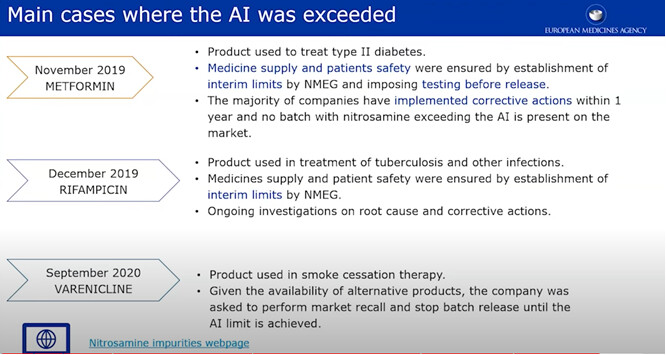
Although expected to be based on a risk/benefit evaluation (which is unknown in this case), the temporary solutions agreeable with the competent authority should be broader than Q&A 22. Think about SAR, MW corrections, LCR level re-evaluation… If the medical need is high and the science well understood and Q&A22 not useful (value-wise that is, the fact that a CAPA plan in the sense of remediation is not possible due to being stuck at ALARP is something else).
A positive Ames test in principle does not prevent in vivo follow-up testing or a readacross exercise. It is always worth considering if you mechanistically understand the outcome of the Ames test and what can be done to improve that understanding if Ames test positivity is evaluated to be unexpected.
I would also look critically again to the risk assessment, did you maybe miss a nitrosamine risk or a root cause? The case sounds like a possible transnitrosation or a multiphase problem, a sort of equilibrium that is not well understood (especially if the identified nitrosating agent source is already minimized).
I believe there should be value in the work that was done to show where the technical limitations are, especially if the medical need is high.
When step 3 remediations are further advancing, I think indeed discussions on the extrapolation of the ICH M7 ALARP principles might come up again with regulators:
“In cases where control efforts cannot reduce the level of the mutagenic impurity to below the
acceptable limit and levels are as low as reasonably practical, a higher limit may be justified based on a risk/benefit analysis.”
For low CPCA categories and high MDD applications, the ALARP level might be significantly higher than the limit based on the state of the art remediation efforts in some cases.
It is better to take the follow-up assay and understand the real mutagenicity, if the levels could not be controlled.
This is also important if multiple impurities detected and there is challenge in meeting total impurity limit as regulatory agencies either expect to control total impurities based on most potent impurity or calculated excess cancer risk that does not exceed 1:100,000.
With combination product, meeting total impurity limit is challenging and required consideration from regulatory agencies.