Must read from the team at Merck, including @schlinjo1975. Hopefully, this can define the limit for all -olols.
Thank you Jason, we are really proud of this one. And a big thank you to our first author Stephanie Simon. It appears that she’s not yet on the Nitrosamine Exchange - I’ll send her an invitation right away.
Below are the highlights, but we enourage you all to read the hole thing, it’s open access ![]()
• First presentation of in vivo mutagenicity data for an NDSRI of a beta-blocker
• Nitroso-bisoprolol was not mutagenic in standard and modifed Ames tests using rat or uninduced hamster S9, but positive in EAT with 30 % induced hamster S9
• ecNGS revealed a low mutagenic potential, suggesting that nitroso-bisoprolol is not in the cohort-of-concern
• Using benchmark dose analysis, new safe limits far above the published 1.5 µg/day could be derived, suggesting that the CPCA based on SAR considerations solely is over-sensitive and should not be capped at the TTC.
• The new safe limits for one beta-blocker determined in the present work may serve as blueprint for class-specifc PDE or AI to be applied to NDSRIs that bear an isopropyl or tert-butyl group connected to the nitroso group.
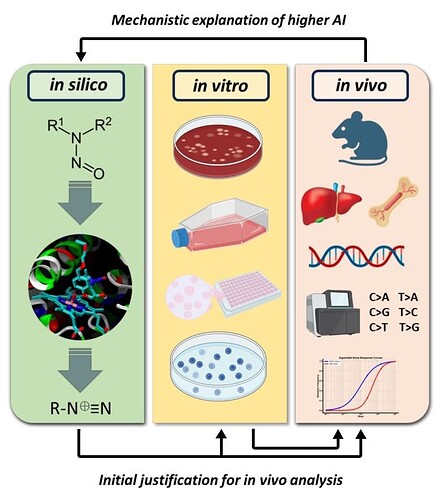
• Horizontally integrated in silico–in vivo analysis shows good agreement between CADRE (QM) outcomes, augmented here with CYP-docking analysis, and in vivo results
• In silico (QM) models can be used a priori to gauge feasibility of a higher AI and/or post in vivo studies to gain greater confidence in the proposed limit based on mechanistic interpretation
excellent work Joerg, congratulation to you and to the team!!
Just a question, do you consider the presence of the isopropyl (or tert-butyl group) next to the amine as the responsible factor for the very low toxicity of the ‘’-olols’’ or the presence of the hydroxyl group in beta thesis?
thank you,
Christos
Hi Christos,
from what I understand about SAR I’d say that both are equally important. However, the hydroxyl group in beta position appears to be a common feature of all beta agonists and antoagonists, whereas isopropyl and tert-butyl are only present in some (I’ve counted 17 of 25). The hydroxyl group being a common feature was the reason for not pointing it out explicitely.
looks reasonable, thanks Joerg!!
The hydroxyl group has been a pet theory of mine for a while, ever since the FDA first published the poster of their AMES test data on various molecules.
I have never seen anything to back it up though before, just a single data point from 1 API.
Is this the proof I have been searching for?
Dear Mark,
for more information on this please look at the following position paper
hope it helps,
best regards
Christos
Dear Jason, I’m happy that you find our work important (as we do ![]() ). I think, it’s important to mention that we are Merck Healthcare KGaA, to avoid confusion with Merck (MSD). It is one of our goals to get acceptance of our approach as a class-specific AI for “olols”-
). I think, it’s important to mention that we are Merck Healthcare KGaA, to avoid confusion with Merck (MSD). It is one of our goals to get acceptance of our approach as a class-specific AI for “olols”-
Dear Simon,
Many thanks for your extremely detailed and useful article for N-nitroso bisoprolol. I would like to ask you, do you have any positive experience from the agency regarding this matter?
Thank you in advance.
Dear Marina, I’m happy to say that some regulators very much appreciate our data package. Currently, our nitroso-bisoprolol case is been discussed within agencies, but we don’t have any decision or reaction yet. Best, Stephanie
Many thanks for your prompt reply. I hope for positive feedback from agencies.