Povidone (polyvinylpyrrolidone, PVP) is a commonly used excipient in the formulation of different pharmaceutical dosage forms. It serves as a suspending and dispersing agent, as well as a binding, granulating, and coating agent for tablets.
Nitrites present as impurities in excipients are the primary source of nitrosating agents that can lead to the formation of nitrosamines1. In this process, the rate-limiting factor is usually the trace levels of nitrite, rather than the more abundant secondary amine. This analytical note describes
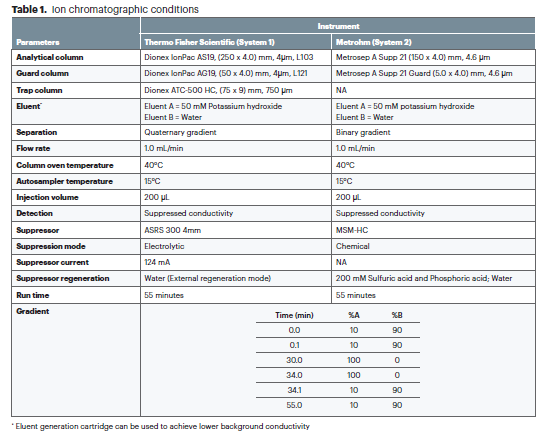
a selective and sensitive ion chromatography procedure for determining nitrite and nitrate levels in povidone. The method utilizes anion exchange separation coupled with conductivity detection.
Limit of Quantitation (LOQ): 0.2 μg/g for nitrite and
1.2 μg/g for nitrate with respect to 25 mg/mL sample
concentration
Validated Range: 0.2 μg/g - 20 μg/g for nitrite and 1.2
μg/g - 40 μg/g for nitrate with respect to 25 mg/mL sample
concentration.
Precision: %Relative standard deviation (RSD) of nitrite
and nitrate of the 6 recoveries at LOQ levels were less than
20%
Accuracy: %Recovery for nitrite at 0.2 μg/g, 4 μg/g, 20
μg/g and nitrate at 1.2 μg/g, 8 μg/g, 40 μg/g were within
100 ± 30.0%
@mayank.bhanti @Mrunal @SureshP
USP Application Note:
Nitrite and nitrate in povidone_AppNote v03.pdf (1.8 MB)
Disclaimer
This information is intended to serve as a resource for informational purposes only and not as an USP-NF compendial documentary standard. USP shall not be responsible to verify the accuracy, completeness, timeliness, quality, and correctness of the information developed and/or posted by its members. This document is on an “as-is”, “as available” basis and USP expressly disclaims all warranties, including the warranties of merchantability, fitness for a particular purpose, and non-infringement. This document does not reflect USP or USP’s Expert Body opinions. Parties relying on the information in this document bear independent responsibility for awareness of, and compliance with, any applicable federal, state, or local laws and requirements.”
“By participating in discussions and posting content within the Analytical Hub, you confirm your agreement to the original Terms of Service of the Nitrosamines Exchange Community. We remind all members to respect intellectual property rights when sharing information, data, or resources. This includes but is not limited to: 1) Avoiding unauthorized distribution. 2) Crediting original sources. 3) Protecting proprietary information.”