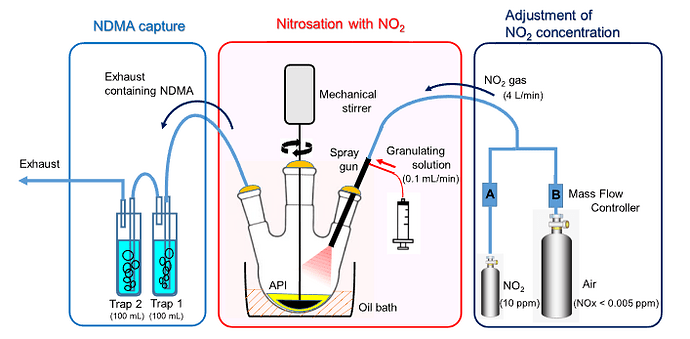
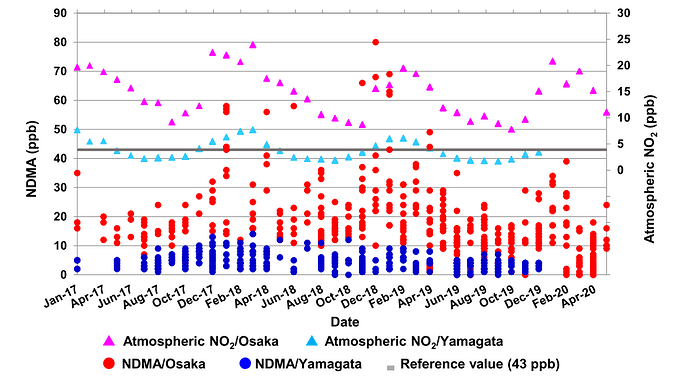
This research has illustrated that a trace amount of atmospheric NO2 can induce the nitrosation of dimethylamine (DMA).
This holds particular importance in processes like fluidized bed granulation, where substantial quantities of air and heat co-occur.
Experimental models have confirmed that DMA, present in limited amounts within the metformin API, reacts with NO2 at a concentration range akin to the intake air of an actual fluidized bed granulator. This reaction results in the formation of N-nitrosodimethylamine (NDMA).
Furthermore, the quantity of NDMA generated exhibits a positive correlation with the NO2 concentration, DMA content, reaction time, and reaction temperature.
In this specific scenario, it was determined that the influence of nitrite in the excipient was minimal.
What are your thoughts on these findings?