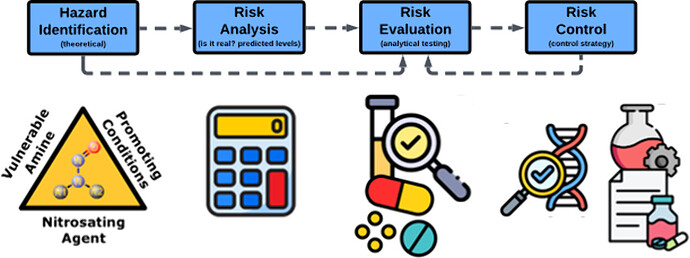
Dr. Olivier Dirat et al. presented a paper on the workflow for risk assessment of nitrosamines in medicines.
https://pubs.acs.org/doi/abs/10.1021/acs.oprd.5c00097
ABSTRACT
The ICH M7 guidance “Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk” requires that drug substances and drug products be assessed for potential mutagens including N-nitroso compounds. N-Nitrosamines are a class of N-nitroso compounds within the cohort of concern as defined in ICH M7 because they can display carcinogenic potency with acceptable intakes in the range of ng/day rather than μg/day. Therefore, control to levels below the ICH M7 threshold of toxicological concern may be required. Following the detection of certain simple N-nitrosamines in marketed medicines between 2018 and 2019, health authorities requested that marketing authorization holders assess all their products (both chemical entities and biologics) for the presence of N-nitrosamines. It became important to establish scientifically robust and reliable approaches for the assessment of small molecule drug substances and products for the potential presence or formation of N-nitrosamines. Included are workflows and associated guidance to enable the reader to risk assess drug substances and drug products that contain chemical entities for the potential presence or formation of N-nitrosamines. These workflows and guidance are the culmination of five years of cross industry collaborations. They are based on known and emerging risk factors for the presence and formation of N-nitrosamines. In combination with regulatory guidance, these are considered as a “best practice” within the Pharmaceutical Industry for conducting drug substance and drug product assessments.