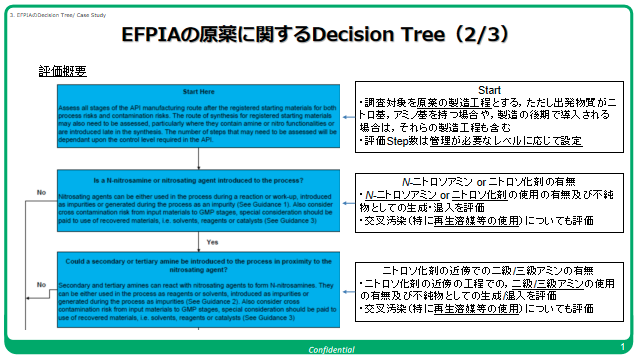
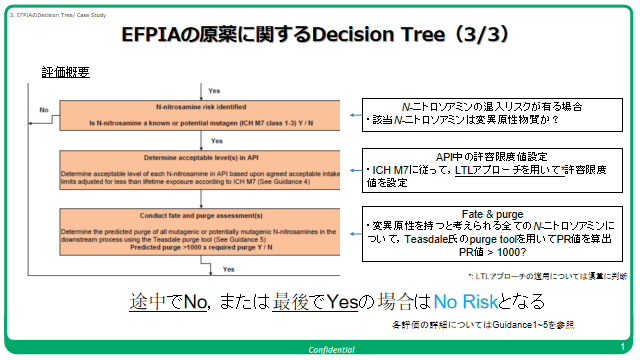
In the last few days, I have been hearing a lot of noise about risk assessment and the robustness of the assessment in recent cases of Nitrosamines.
I thought it would be a good idea to bring back the EFPIA Workflows for quality risk management of nitrosamine risks in medicines published in December 2020. Several of our community members were part of the team that put together the guide @AndyTeasdale included!
I have taken the liberty of producing non-official translated versions in Spanish, Portuguese, Japanese, and Korean of the guide using google translator, so I can not hold liable for the accuracy of the translation.
Korean: workflows-for-quality-risk-management-of-nitrosamine-risks-in-medicines KOR.pdf
Japanese: workflows-for-quality-risk-management-of-nitrosamine-risks-in-medicines JAP.pdf
Portuguese: workflows-for-quality-risk-management-of-nitrosamine-risks-in-medicines POR.pdf
Spanish: workflows-for-quality-risk-management-of-nitrosamine-risks-in-medicines ESP.pdf