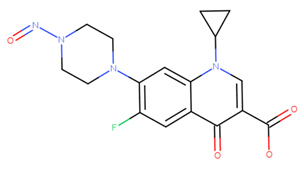
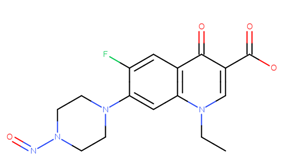
Dear Yosuke, many thanks for your information.
Do you have identify the corrections which have been done in this update?
kind regards
Christos
Thank you for asking, Cristos. I compared both Excel. Smiles and structure of N-nitroso-tizanidine Impurity 3 differed from each other.
In addition, the K Line(First publication date) was described as the “publication date” in the old version. Due to this change, the K line (first edition publication date) of 21 nitrosamines has been changed from the previous version.
thanks a lot for your time Yosuke, it is much appreciated!!
EMA Appendix 1 was updated on 5 February 2025. Twelve nitrosamines are added to the list.
| Nitrosamines | CPCA Cat. | AI (ng/day) |
|---|---|---|
| 2-(N-nitrosomethylamino)-N-[6-(trifluoromethoxy)-1,3-benzothiazol-2-yl]acetamide | 2 | 100 |
| N-nitroso-dalbavancin | 4 | 1500 |
| N-nitroso-dasatinib | 5 | 1500 |
| N-deshydroxyethyl-N-nitroso dasatinib | 3 | 400 |
| N-nitroso-desmethyl-cabergoline | 1 | 18 |
| N-nitroso-desmethyl-lercanidipine | 2 | 100 |
| N-nitroso-desmethyl-lercanidipine impurity D | 2 | 100 |
| N-nitroso-N-desmethyl-mianserin | 3 | 400 |
| N-nitroso-nirogacestat | 1 | 18 |
| N-nitroso-norfloxacin | - | NMI* |
| N-nitroso-sarcosine | 4 | 1500 |
| N-nitroso-vancomycin | 4 | 1500 |
*Limit derived using structure-activity-relationship (SAR)/read-across approach using the TD50 of n-nitroso-ciprofloxacin as point of departure.
N-nitroso-ciprofloxacin
N-nitroso-norfloxacin
TD50 of n-nitroso-ciprofloxacin? ![]()
I am scratching my head right now. Do someone really did a Carc. test for N-Nitroso Ciprofloxacin? Good thing is that similar structures (some piperidine like ones perhaps too?) would be able to read across from that TD50?
I checked, the argumentation line in N-Nitroso Ciprofloxacin and still says -tive in In-vivo mutagenicity test.
I think this is just a typo. ![]()
Dear @Yosukemino,
In this EMA update, “other N-nitroso-structures” were added, some derived from aromatic heterocycles such as indole.
I would like to know if the community is already including these compounds in theoretical risk analyses through kinetic simulations.
In addition, synthesizing many standards of these compounds can be challenging, due to the specific synthesis and stability conditions
Thank you in advance.
Dear colleague,
If I may say, we also thought that N-nitrosated indoles, should not be formed mainly by chemistry aspects. However, in NAP tests we have seen the N-nitroso formed as well as the C-nitroso. The N-nitroso when developing a method seems to be formed in most sample/standard procedures in the blink of an eye as per air exposure. How this translates to formation in drug product is still to be seen. But any drying process may be risky. In kinetics calculations is difficult to include the NOx contribution into the equation and make a reliable statement when the possible main nitrosating agents source is in air and first a quantification of how much is present is needed.
At the end it may depend on the regulatory point of view. From others shared experiences here, you may say “not formed” and then the authority says, but analytical standards are available. Or will requiere experimental investigation. You will never know until you have your risk assessment pressure tested by an authority.
Dear Diego,
I appreciate your information and discussions on the subject.
What we have seen is that many standards are available for purchase, but many of them are not synthesizable.
Our colleague @Naiffer_Host discussed this issue a while ago.
In the case of indoles, for example, the most activated position and susceptible to reaction is in the ring itself. But as you mentioned in relation to exposure to air, it is somewhat complicated.
I look forward to other experiences that can be shared here and/or published articles.
I would like to note that based on theoretical considerations, 3-substituted indoles are prone to form N-nitrosamines and the structures mentioned in EMA’s list are of this type.
We have asked a nitrosamine standard supplier for a nitrosamine derived from an indole type structure and we have got a NAP type study showing that the traces of the isolated reaction product was not in fact N-nitrosamine.
@Diego_HM what type of indole structures have you tested? It is to understand that during sample preparation, by contact with air in solution or in solid phase, these products are so reactive with NOx from air?
Thanks for your question:
First some background: Indoles as you all know react first at C3 if substitued C2 and only in the benzene ring in special circunstances. The N-H is heavily prone to electrophylic substitution.
I could not share which compound or family of compounds, but it was interesting as the compound was already substitued at C3 and C5. Leaving only C2 unsubstitued. Reaction of indoles and C3, C2 substitued indoles seems to be very complex as highlighted by Reactions of indoles with nitrogen dioxide and nitrous acid in an aprotic solvent - Organic & Biomolecular Chemistry (RSC Publishing). However, 3 alkyl substitued indoles could form N-Nitroso compounds based on this study but stability is an issue and perhaps why the N-nitrosated indole is so reactive.
Additionally, in our studies the N-nitroso seemed to form very easily in with certain solvents at certain pH with the API in solution. While more stable in others w/o a significant increase from base. But, you leave the solution in the bench exposed to air and you start seeing quite a bit of by-products, including the N-nitroso regardless of the buffer used after some time. Our marker (standard) by the way needs refrigerated conditions at least or lower °T and by the way aid us to differentiate between the C-nitroso and N-nitroso. Again instability.
Fortunatelly, this reactivity ends in a no issue for N-nitrosated indoles in a case by case approach. But, of course may open a chapter for C-nitrosation. This is highlighted in efpia-nitrosamines-risk-management-workflows-jun-24-udpate.pdf as a risk coming from NAP test where it says: “Where MS confirms the potential presence of a nitrosamine and further action is likely to be required, it is recommended to manufacture or isolate an authentic sample for structural confirmation to rule out potential false positives (e.g. C-nitroso, O-nitroso, oxime, etc.). If significant alternative products (i.e. not the expected nitrosamine) are formed from these screening conditions, then identification of the structures may be considered to understand any potential mutagenicity risk (these impurities can be considered as “reasonably expected” from an ICH M7 perspective).”
Thank you so much for these fascinating details!
This article is very interesting:
The authors showed that even under favorable conditions for the formation of nitrosamines, unsubstituted indoles at C3 would not form these compounds. The proposed mechanism helps in this justification.
This shows that the formation of these compounds in drug produtct may probably be remote.
I would say in general it really depends on where the substitution in the indole is present to understand if a N-nitroso compound could be formed or not and if stable, as highlighted in your shared articicle as well as the one I shared.
Once again nitroso compounds always surprise us.
Thank you for the excellent discussion. How many more times must we be surprised…
Oops, EMA Appendix 1 was updated on 20/02/2025 to remove N-nitroso-nirogacestat. How did this happen? ![]()
Thanks Yosuke for the update.
However, can you guide whether list will update for this change.
Hi Pooja,
The current rev.8 does not include N-nitroso-nirogacestat which was included in the previous rev.8. This may be a correction.
Yosuke
Hello everyone,
I checked the structures named by EMA:
N-nitroso-desmethyl-lercanidipine and
N-nitroso-desmethyl-lercanidipine impurity D
The first one is OK,
but the second one:

should be N-nitroso-desmethyl-lercanidipine impurity C, because impurity C is the only lercanidipine impurity with the aromatic pyridine ring:
C.3-[1-[(3,3-diphenylpropyl)(methyl)amino]-2-methylpropan-2-yl] 5-methyl 2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
Do you agree?
Dear Giovanni,
you are absolutely right, the provided structure and smiles are corresponds to the N-nitroso-desmethyl-lercanidipine impurity C and not D.
Furthermore, i think that soon will be added in the list an input also for the N-nitrosodesmethyl impurity E (N-(3,3-diphenylpropyl)-N-(2-hydroxy-2-methylpropyl)nitrous amide smiles: CC(O)(C)CN(CCC(C1=CC=CC=C1)C2=CC=CC=C2)N=O
Christos