11 NDSRIs are new and 4 NDSRIs are updated. The acceptable limit for N-nitroso sertraline is currently classified as Category 2, the same as that set by Health Canada.
Thanks a lot Yosukemino,
Something awesome I see now is the following:
In-vivo positive TGR data has now been used to define a limit based on the tiers of the CPCA for the following:
- N-Nitroso Sertraline: In-vitro -tive, In-vivo +tive (1500 ng/day to 100 ng/day)
- N-Nitroso Nortryptyline/Amitriptyline (8 ng/day to 18 ng/day)
This is a major update under my perspective, still not in the Q&A but at least it is accepted in a case-by-case.
New NDSRIs
| Name | Source | CPCA | AI(ng/day) | Note |
|---|---|---|---|---|
| N-nitroso-2,4-thiazole amine | Nirmatrelvir/ Ritanovir | 1 | 18 | CPCA-derived AI as substance tested positive in in vivo mutagenicity study |
| N-nitroso-acebutolol | Acebutolol | 4 | 1500 | |
| N-nitroso-buspirone Impurity 1 | Buspirone | 3 | 400 | |
| N-nitroso-desmethyl-dimetindene | Dimetindene | 1 | 18 | |
| N-nitroso-desmethyl-rivastigmine | Rivastigmine | 2 | 100 | |
| N-nitroso-iminodibenzyl | Melipramin | 78000 | Limit derived using structure-activity-relationship (SAR)/read-across approach using the TD50 of NDPh as point of departure. | |
| N-nitroso-maprotiline | Maprotiline | 1 | 18 | |
| N-nitroso-propafenon | Propafenon | 2 | 100 | |
| N-nitroso-valsartan cyano-desvalerylmethylester | Valsartan | 2 | 100 | |
| N-nitroso-vanzacaftor | Vanzacaftor | 4 | 1500 | |
| N-nitroso vildagliptin amide impurity | Vildagliptin | 5 | 1500 |
Updated NDSRIs
| Name | Source | CPCA | AI(ng/day) | Note |
|---|---|---|---|---|
| N-nitroso-ciprofloxacin | Ciprofloxacin | NMI | Substance tested negative in in vivo mutagenicity study. Can be controlled according to ICH Q3B. | |
| N-nitroso-diclofenac | Diclofenac | 78000 | Limit derived using structure-activity-relationship (SAR)/read-across approach using the TD50 of NDPh as point of departure. | |
| N-nitroso-nortriptyline | Amitriptyline, Nortryptyline | 1 | 18 | CPCA derived AI as substance tested positive in in vivo mutagenicity study |
| N-nitroso-sertraline | Sertraline | 2 | 100 | CPCA-derived AI as substance tested positive in in vivo mutagenicity study |
N-nitroso-ciprofloxacin: 1500 => NMI
N-nitroso-diclofenac: 1500 => 78000
N-nitroso-nortriptyline: 8 => 18
N-nitroso-sertraline: 1500 => 100
The AI of some Category 5 compounds may be changed to 78000.
Some pharmaceutical companies may worry that positive results could lower the AI, making them hesitant to conduct in vivo testing.
You are right, but at the end that is the risk, data could go both sides. It is a measured risk.
For the time being I would say if a TGR is planned, the safest bet is for compounds that are Category 1. You would not get a lower limit than 18 ng/day and it may be worth the risk as otherwise, you would not a get so easily a higher limit.
I am still not sure if anybody is doing a full carc. test by OECD 451 standards. As it takes 2 years, if someone is doing it, results may be still not ready that would be the other secure way of having a higher limit than CPCA (or lower who knows).
thanks a lot Yosuke for the update.
So, N-nitrososertraline had a limit of 1500ng/day through a negative Ames test which now decrease to 100ng/day due to a positive in-vivo test!! this is very impressive.
I am not so familiar with the toxicology but as i have understand the positive results in-vivo is not always ‘‘bad’’ results as sometimes it could be used for setting higher limits through the Benchmark dose approach.
thank you
furthermore i would like to highlight the entry of the N-nitroso-valsartan cyano-desvalerylmethylester. It should be noted that its cas number is wrong. This cas number (2407652-86-0) is for the corresponding carboxylic acid and not for this reagent. Also, the IUPAC name which is given is not right. If it is placed in the Chemdraw software, the structure which is showed is not the N-nitroso-valsartan cyano-desvalerylmethylester. Overall, quite careless work
thank you
I think even the regulatory agencies would not recommend running a Carc study based on the principles of the 3Rs. I remember some discussions on trying to extrapolate a limit from in vivo mutagenicity studies but not sure if anyone is actually doing this, as EMA is relying on CPCA limits for positive in vivo studies.
@conudel @jbercu @AndyTeasdale can maybe share any updates on deriving a limit based on in vivo studies and if this has been discussed with regulators?
Just a minor comment here, I have read and being told from a colleague in this forum, that someone was doing a carc. study. If that is still the reality, I cant say.
For the Mutagenicity studies for carcinogenicty end-points a lot of discussion have been on-going around the topic. My personal opinion is that the topic is going well. But, we have here the colleagues that are moving the topic forward to share further details ![]()
Hi @chrischar,
I want to remind you that the Japanese government did not accept Lilly’s approach which assigned an AI of 4400ng/day or 1500ng/day (not COC) for N-nitroso atomoxetine from BMDL in in vivo test and other evidence. The points are as follows;
- N-nitroso atomoxetine is classified as class 2 under ICH M7 because it is positive in in vitro and in vivo mutation tests. The maximum AI for class 2 is 1500 ng/day.
- ICH M7 Q&A #7.2 explained that in vivo gene mutation assays alone are currently not validated to directly assess cancer risk because the endpoint is mutation and not carcinogenicity (i.e., they are used for hazard identification)
- The FDA, EMA, and TGA have announced that the AI of N-nitroso atomoxetine is 100 ng/day, but there is currently no information available on any recalls or other measures being taken.
As far as I know, no regulatory authority has accepted this innovative approach. It will probably take some time before the AI calculation from in vivo test is accepted.
Yosuke thanks a lot for your feedback.
I agree with you, it will take time before the AI calculation from in vivo test could be accepted but i think that the train is on the rails ![]()
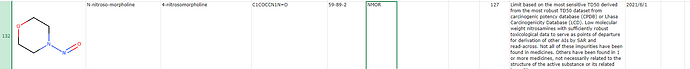
Can anybody find the limit for NMOR i.e. N-Nitrosomorpholine in the new released appendix?
Thank you @Yosukemino
Yes, it is there in the previous versions of appendix probably by EMA, but it is not mentioned in the recent appendix released by US authorities with the September update of the guideline. The US site has categorized the nitrosamines in two parts viz Table 1 contains the recommended AI of NDSRI or related substances based on CPCA and Table 2 based on the toxicoligical data of nitrosamines or read across with a surrogate with robust toxicology data (here NDMA & NDEA are listed). But I was not able to find NMOR i.e. N-Nitroso morpholine in any of these tables on the recently update relevant US site.
The EMA AI for NMOR (127 ng/day) is based on the most sensitive TD50 derived from the most robust TD50 dataset from carcinogenic potency database (CPDB) or Lhasa Carcinogenicity Database (LCD).
It should be accepted also by FDA without objections.
Here the point is that whether USFDA has completely missed out NMOR from the new list?
NMOR is neither listed in Table 1 nor in Table 2.
While FDA is gradually merging some otherwise communicated RAILs (e.g. from guidance docs or specific FDA webpages) to the tables mentioned, this exercise is indeed not per se complete.
Based on mentioning NMOR as surrogate candidate based on robust data in “Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) Guidance for Industry”, one can derive endorsement of the available NMOR data and thus consider acceptability of 127 ng/day (like published at EMA/HC/TGA level).
USFDA AI list did not include NMOR before, I guess. The AI list included nitrosamines with AI determined by CPCA in table 1 and nitrosamines with AI determined by read across in table 2, respectively. NDMA and NDEA are now in table 2 as nitrosamines with AI determined by compound-specific carcinogenicity.
NMOR is not included in revision table. NMOR was not excluded, it was just not included in the first place.
I agree with @ccdw and @Yosukemino. However, I don’t understand that when NMOR is having a robust carcinogenicity data like NDMA & NDEA and also already published at EMA/HC/TGA, then why USFDA is skipping this NMOR nitrosamine from the list.