@Pradpharma and @ccdw , I believe this case is an example/widening of what is described here (Q&A 10 B3): ‘If a surrogate nitrosamine is available with sufficiently robust carcinogenicity data, the TD50 from the surrogate substance can serve as a point of departure for derivation of AI by SAR and read across.’
Thanks @lucas10mauriz. To some extent I agree. But ideally we do not have any robust carcinogenicity data and TD50 here.
All i observe here is, a clear non-mutagenic evidence from an in vivo assay and same is used for structurally relevant molecule.
I feel this could be a step forward for picking all structural features for NDSRIs that turned negative for mutagenicity for in vivo assay and then try to apply them, if it permits- This could be great help in saving time and money for NDSRIs where this can work out.
Yes, if we don’t perceive it as a widening, I see it as the next step that we’re taking, as you mentioned
In vivo mutagenicity data is not equal to the described carcinogenicity data in the guidance, cf. also the lack of well-established usability of positive in vivo mutagenicity data (potency ranking, establishing carcinogenicity-mutagenicity relation for nitrosamines with regulators).
Scientifically I’m of course open to a case-by-case justified teleological interpretation of the guidance rather than a literal one (by means of non-binding precedent opens up this type of interpretation for other guidance elements as well!), but procedurally/practically I’m wondering if the recent in vivo mutagenicity data have to go back to NITWG for re-assessment (now as option Q&A 10 B3 case instead of B4 case) or if this is a newer option B5 possibly not requiring (re-)attempted international harmonisation (e.g. if under option B3 full alignment didn’t work on the reference, does it make sense to require time for a reloop for the “B5” type of use).
cf. EMA (2023). Sixth Nitrosamine Implementation Oversight Group (NIOG) - meeting with pharmaceutical industry. Thursday, 07 December 2023. Sixth Nitrosamine Implementation Oversight Group (NIOG) - meeting with pharmaceutical industry | European Medicines Agency; https://www.ema.europa.eu/en/documents/presentation/presentation-update-new-developments-nitrosamines_en.pdf
I want to ask for the opinions of @conudel because he is the author of the EFPIA position paper. What do you think about the AI of N-nitroso lisinopril determined as NMI, Raphy?
This is a very interesting development because it seems like the EMA have accepted for the first time the use of SAR and physiochemical properties to set an NMI for an NDSRI. What I am curious about is why this was done only for nitroso-lisinopril and not for all the other nitrosamines of ACE inhibitors (-prils). It again seems like they selectively chose one compound out of a group and applied a new methodology. I wish they would have been more transparent and given a more detailed explanation for this new limit.
Thank you for sharing your thoughts, Raphy. We need further information about this case from EMA. I added the calculation results by MarvinSketch. The hydrophilic property looks important in addition to local similarity.
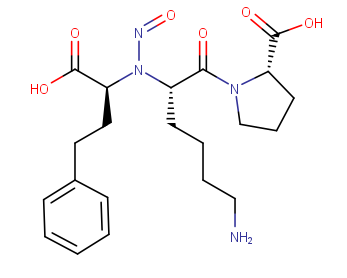
N-nitroso lisinopril
Smiles: NCCCC[C@H](N(N=O)[C@@H](CCC1=CC=CC=C1)C(O)=O)C(=O)N1CCC[C@H]1C(O)=O
CPCA: Cat.5 (one alfa-hydrogen on both sides of N-nitroso group)
Mw: 434.49
log P of noniononic species = 1.84
Solubility in Water
Solubility category: High (higher than 0.06 mg/ml)
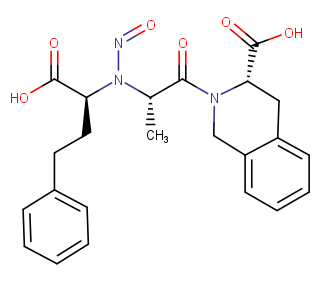
N-nitroso quinaprilat
Smiles:C[C@H](N(N=O)[C@@H](CCC1=CC=CC=C1)C(O)=O)C(=O)N1CC2=CC=CC=C2C[C@H]1C(O)=O
CPCA: Cat.5 (one alfa-hydrogen on both sides of N-nitroso group)
Mw: 439.47
log P of noniononic species = 3.33
Solubility in Water
Solubility category: Low (lower than 0.01 mg/ml)
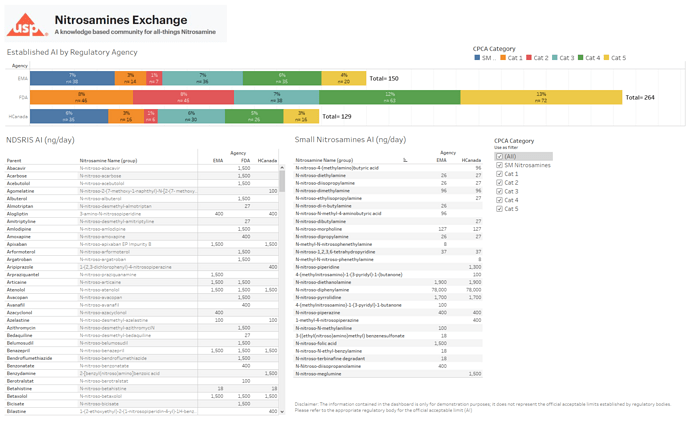
A contribution to this post… with inspiration from Yosuke and Lucas, I put my data analytics hat to build a dashboard than can be useful to identify and keep track all of the AI limits published. I’m working internally to get approval to publish the dashboard on a public domain for easy access to everyone.
If I recruit a group of contributors to the data set and make any updates that are published on the website, we can really build a great tool!
Here is a sample.
That’s fantastic to hear! Your initiative to build a dashboard for tracking AI limits is not only commendable but also incredibly valuable for the community. With a group of contributors working together, there’s no doubt that you’ll create an invaluable resource for all.
Lucas
I received the answer about Appendix 1 of the EMA Q&A at the PDA symposium in Japan. Dr. Robert Bream kindly answered my question. It helps to understand what nitrosamines are included in Appendix 1. Not all nitrosamines in Appendix 1 have been detected in the drug products at a level above LOD before. Please check it.
Q. Should all impurities in Nitrosamines Q&A Appendix 1 be controlled to avoid exceeding acceptable intakes? Tamoxifen is used in breast cancer treatment. Nitroso-ticagrelor may be unstable due to isoxazole formation from cyclopropane ring opening. Some nitrosamines in the list look inappropriate. How are the nitrosamines in the Appendix 1 determined?
A. Yes, all nitrosamines should be controlled according to the published AI. For APIs indicated for advanced cancer ONLY (ICH S9 scope), then ICH Q3A/B limits apply. N-nitroso-tamoxifen has been included in the appendix as tamoxifen is also used in non-ICH S9 cancer indications and in non-cancer indications. Nitrosamines that have been detected during confirmatory testing and/or for which the MAH, CHMP and/or NCA (national competent authority) has requested the establishment of an AI be included in Appendix 1. It could be possible that Appendix 1 contains NDSRIs, especially those that have been derived using the CPCA approach, that have not been detected or for which the levels measured were below 10% of their AI. The publication of the CPCA derived AIs is considered beneficial to make MAHs aware of the possibility of the formation of a nitrosamine impurity and their acceptable limit to enable them to develop an analytical method that is sufficiently sensitive.