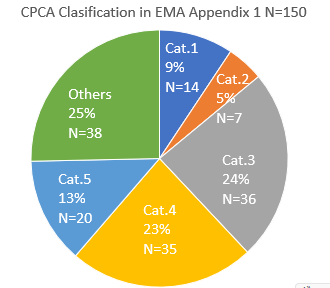
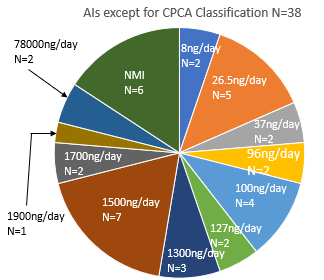
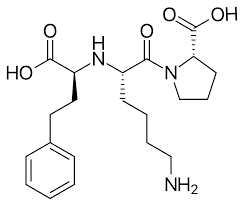
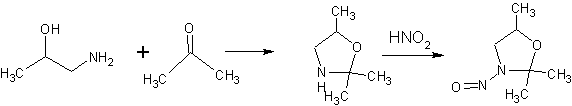Have you checked the update to the AI list (Appendix 1: Acceptable Intakes Established for N-nitrosamines, Reference Number: EMA/72902/2024 Rev. 4)?
2-(4-nitrosopiperazin-1-yl)ethanol - 400 ng/dia (CPCA 3)
3-((ethyl(nitroso)amino)methyl) benzenesulfonate - 18 ng/dia (CPCA 1)
4-nitroso-methyl piperazine-1-carboxylate - 400 ng/dia (CPCA 3)
N-(2-hydroxy-2-phenylethyl)-N-(4-nitrophenethyl)nitrous amide - 400 ng/dia (CPCA 3)
N-(4-aminophenethyl)-N-(2-hydroxy-2-phenylethyl)nitrous amide - 400 ng/dia (CPCA 3)
N-nitroso-articaine - 1500 ng/dia (CPCA 4)
N-nitroso-desmethyl-edoxaban - 100 ng/dia (CPCA 2)
N-nitroso-fenfluramine - 400 ng/dia (CPCA 3)
N-nitroso-folic acid - 1500 ng/dia (CPCA 4)
N-nitroso-furosemide - 1500 ng/dia (Limit based on negative bacterial reverse mutation test)
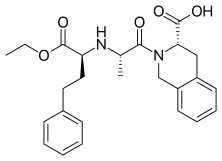
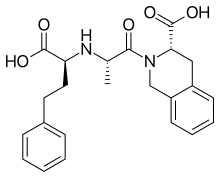
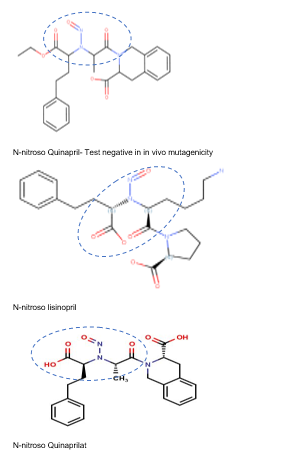
N-nitroso-lisinopril – NMI (Limit derived using structure-activity-relationship and physicochemical features of N-nitroso-lisinopril, N-nitroso-quinapril and N-nitroso-quinaprilat)
N-nitroso-N-desmethyl-dextromethorphan - 1500 ng/dia (CPCA 5)
N-nitroso-N-ethyl-benzylamine - 18 ng/dia (CPCA 1)
N-nitroso-posaconazole Impurity 1 - 400 ng/dia (CPCA 3)
Rivaroxaban Nitroso Impurity 1 - 1500 ng/dia (CPCA 4)
Rivaroxaban Nitroso Impurity 2 - 1500 ng/dia (CPCA 4)
Rivaroxaban Nitroso Impurity 5 - 1500 ng/dia (CPCA 4)
N-nitroso-trientine Impurity 1 - 18 ng/dia (CPCA 1)
N-nitroso-vibegron - 1500 ng/dia (CPCA 5)
https://www.ema.europa.eu/en/documents/other/appendix-1-acceptable-intakes-established-n-nitrosamines_en.xlsx