Dear @Diego_HM,
Thank you for sharing the information. I’m so excited to read it. It’s amazing!!
According to the revision history, the updates are as follows;
Amendment to Q&A 10 to include the Carcinogenic Potency Categorization Approach (CPCA) and the enhanced Ames test (EAT) for establishing AIs for N-nitrosamines. Addition of Appendix 1, listing the nitrosamines for which AI have been established by the Non-clinical Working Party (NcWP), including new AIs for N-nitrosamines determined using the CPCA. Addition of Annex 2, describing the Carcinogenic Potency Categorization Approach for N-nitrosamines. Addition of Annex 3, describing the Enhanced Ames Test Conditions for N-nitrosamines.
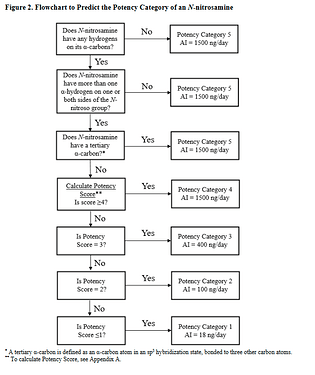
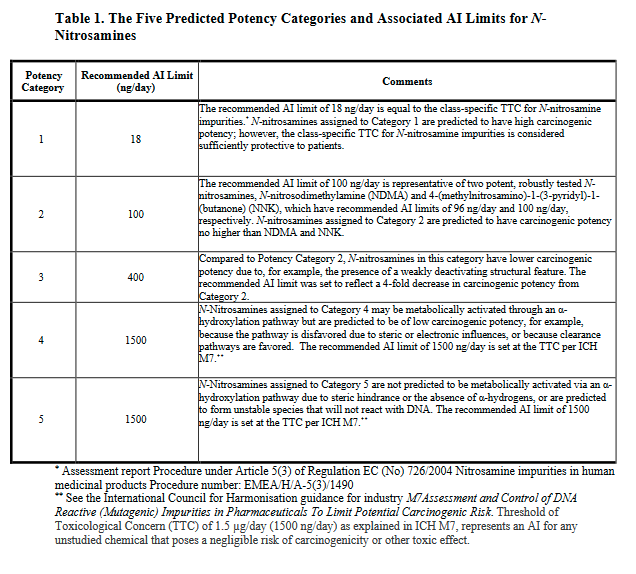
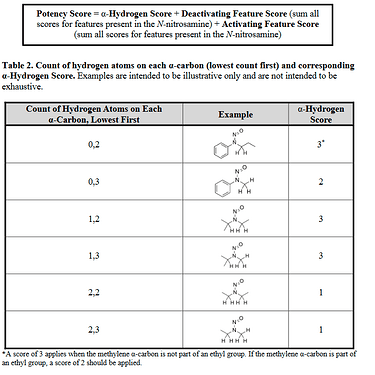
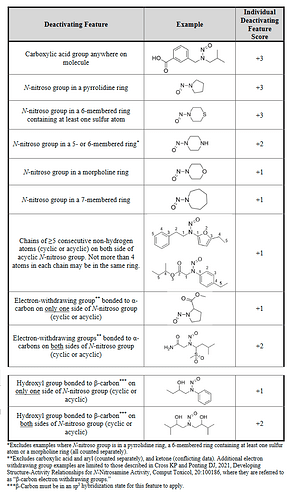
Now we can classify nitrosamines into 5 categories. Here we can count alfa-hydrogen and calculate the “Potency score” from the “alfa-Hydrogen Score”, “Deactivating Feature Score” and “Activating Feature Score”.
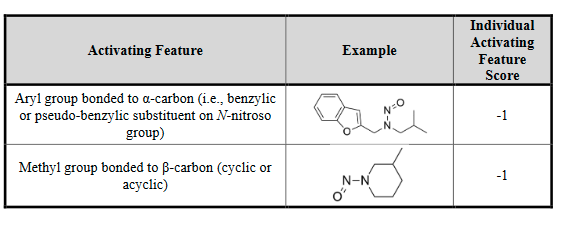
And the enhanced Ames test condition is also available. A negative result in an GLP-compliant enhanced Ames test (EAT, Annex 3) allows control of the N-nitrosamine at 1.5 μg/day. For substances testing positive, the AI should be established using options 1(category approach) or 3(read-across method).
These approaches will help all patients, pharmaceutical companies, and regulators. Let’s enjoy the journey to great science!!