Dear @David
Thank you for your helpful and informative explanation. Of course, I remember you helped us in this community many times. And I want to illustrate your explanation to understand nitrosamines deeply.
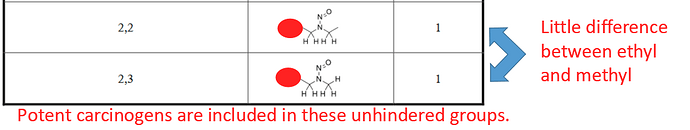
If the most-hindered side is CH2, then whether the right-hand side is CH2 or CH3 makes little difference, both sides can react and highly-reactive diazonium ions can form, hence the score of 1 - reflected in a number of potent carcinogens that match this unhindered pattern.
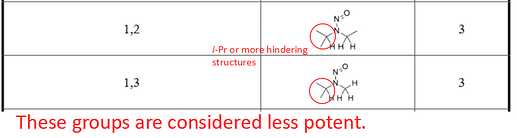
Isopropyl groups, as I have been saying for a number of years, lead to a dramatic reduction in potency, hence the score of 3 for either (1,3) or (1,2).
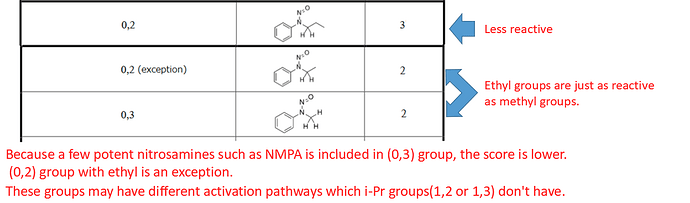
Aryl groups are interesting, because there are a few potent examples such as nitrosomethyl aniline (NMA or NMPA) which explains the lower score for (0,3), and ethyl groups are just as reactive as methyl hence the exception in (0,2). All other groups (other than activating groups) are less labile, making the rest of (0,2) have a score of 3. These also have some potential alternative mechanisms not available to the isopropyl ones, hence the lower scores for NMA and nitrosoethylaniline and ring-substituted derivatives.
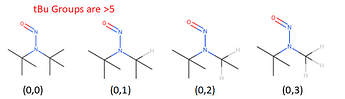
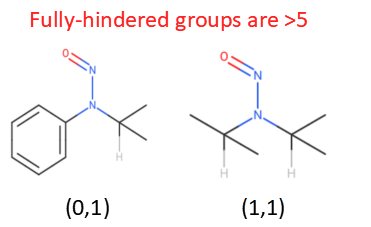
As a caveat, tert-butyl groups [(0,0) (0,1), (0,2), (0,3) where the 0 is sp3], as well as the fully-hindered (0,1) and (1,1) have an effective CPCA score of >5, enough to make them category 4 if they did need the CPCA but of course don’t need it, and the only (3,3) is NDMA - which of course has a compound-specific limit. This is why not all permutations are in the CPCA.
If I made a mistake, please point it out.