Enclosed is the 4th Rev of Questions and answers for marketing authorisation holders/applicants on the CHMP Opinion for the Article 5(3) of Regulation
(EC) No 726/2004 referral on nitrosamine impurities in human medicinal product published
by EMA on 29th June 2021
@DAB Thank you DAB. It is very helpful. Following two are updated.
3. For the ‘call for review’ for chemically synthesised and biological medicinal products, when and how should MAHs report steps 1 and 2 to competent authorities?
10. Which limits apply for nitrosamines in medicinal products?
For former new section addressing line extensions and variations applications is added, and for latter the limit of NMOR is added, according to the article.
Thankyou @Yosukemino.
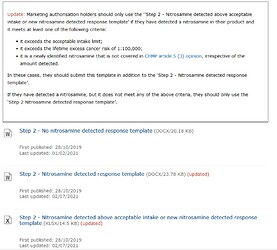
I want to add the information of EMA step2 template which was updated after the update of Q&A. EMA distinguishes how to deal with unknown nitrosamines from known ones. When unknown ones are detected, additional template of excel sheet will be required aside from regular template, irrespective of the amount detected.
Thankyou @Yosukemino for sharing the revised templates here.
Enclosed article provides more details on the same:
https://www.gmp-compliance.org/gmp-news/updated-templates-for-reporting-nitrosamine-contamination-to-regulatory-authorities?utm_source=Newsletter&utm_medium=email&utm_campaign=ECA+GMP+Newsletter+-+2021+-+KW29+-+PEU
Thank you for the additional information. It’s very helpful!!