Has anyone encountered a similar issue?
In Annex 3 of the latest update of Q&A it is stated that the enhanced Ames test must contain 2 N-nitrosamine positive controls. 3 examples of N-nitrosamine positive controls are listed in the Annex: NDMA, CPCA and a NDSI.
What is the criteria for selecting N-nitrosamine positive controls? Is it the environment around the nitrosamine group in NDSRI? The most sensitive strains for identifying the mutagenicity of NDSRIs?
Furthermore, according to OECD 471, can we exchange the strain TA1537 with TA97/TA97a also for testing NDSRIs?
Thank you for answering!
Hi @smotina
I am in opinion the environment around the nitrosamine group in NDSRI could be better criteria for selecting Positive control.
Good question on exchange of strain. Not encountered this.
I do not see any scientific flaw in exchanging strain of TA1537 with TA97a, but how agency perceives it- We may need to wait and watch by making an attempt!.
Although both TA1537 and TA97/97a gives a clue for frameshift mutation, there is slight difference among them pertinently target mutations and presence of R factor plasmid pKM101 in TA97a, that makes it more sensitive than TA1537.
1 Like
@Pradpharma thank you for your answer!
Q&A states that N-nitrosamine positive control needs to be justified based on the anticipated metabolism of N-nitrosamine and the P450 enzymes most likely involved. Based on this I assume that we need to find an appropriate N-nitrosamine positive control that represents the environment of the N-nitroso group in the N-nitrosamine with sufficient data regarding P450 metabolism.
However, identifying appropriate N-nitrosamine positive control with Ames mutagenicity data will take time. Many N-nitrosamines (suitable for positive controls) are not available for purchase, are available only in small quantities and therefore expensive. Furthermore, it is almost necessary to test it on a bacterial test system prior the performance of the Ames assay.
There are several points to think about. Does N-nitrosamine positive control needs to be positive in an Ames test in the presence of metabolic activation only ? Does N-nitrosamine positive control needs to have also carcinogenicity data? Consequently, the range of positive controls is rapidly decreasing.
In case of bacterial strains, many institutions have replaced TA1537 strain with TA97/TA97a strain precisely due to better sensitivity for the detection of mutagens in the latest. In the Q&A, however, they do not highlight this option.
2 Likes
fyi this is being actively discussed across industry at present and it is fair to say there are concerns both in terms of the N-Nitrosamines selected and indeed over the value of a positive control. I would advise to watch this space…
6 Likes
Thanks @smotina for bringing up sensible points.
Availability with certain purity of suitable positive control for each NDSRI is really challenging!
Ideally Yes, N-nitrosamine positive control needs to be positive in an Ames test in the presence of metabolic activation only.
Does N-nitrosamine positive control should have carcinogenicity data? This looks interesting. There are few nitrosamines that were positive in Ames but non-carcinogenic or weakly carcinogenic in rodent studies. So translation of Ames positive to in vivo carcinogen is far job!
As @AndyTeasdale suggested…We will wait and watch Thanks!
1 Like
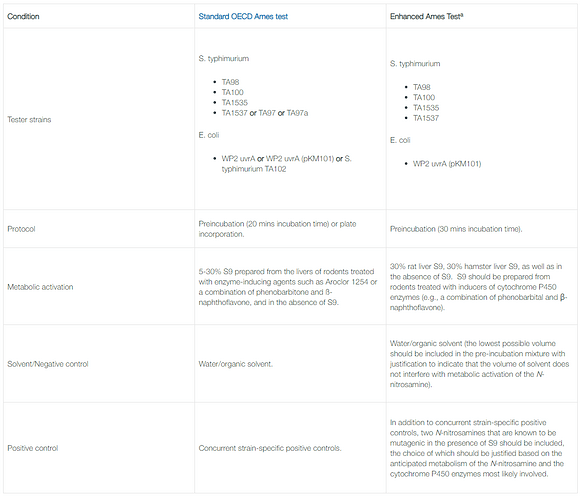
The blog, Current status of the Ames test for N-nitrosamines, shared by Lhasa helps understand EAT. The differences between normal Ames and EAT are summarized in the Table.
2 Likes
As per OECD 471., 5 different concentrations of the test substance should be used in Ames test. Are there any differences in EAT and can anyone clarify which concentrations should be used? thank you very much!
Dear @lovrobucic
The choice of concentrations can be or 5 or more.
Advise: Fix your doses based on cytotoxicity study- That can help you!
@Pradpharma
Thank you very much!
If we consider Enhanced Ames Testing (EAT) according to the EMA/409815/2020 Rev.17 , which exact 5 concentrations would be tested in the EAT? If I understand correctly, If not stated differently in the latest Rev.17, the Enhanced Ames test protocol follows the protocol for the OECD 471. Guideline (of course, with the deviations listed in the Rev.17 such as preincubation, s9 and positive controls)
The maximal tested concentration should be 5 mg/plate and the following concentrations are half log. So, the 5 tested concentrations would be 5, 1.6, 0.5, 0.16 and 0.05 mg per plate?
Thank you!
Dear @lovrobucic,
Please note the concentrations chosen for each nitrosamine/NDSRI shall be specific to it. It is based on their physico-chemical properties and its behavior towards a bacterial cell.
As per OECD, i suggest to do first cytotoxicity, solubility, precipitation tests and then fix your doses to have meaningful interpretation.
All the best!
1 Like