The Japanese government decided on the extension of the deadline for the measurement of nitrosamines and the application for approval of partial change or a notification of minor change from October 31, 2024, to August 1, 2025. The new deadline of August 1, 2025, is the same as the deadline for NDSRIs by the FDA.
The link to the original notification written in Japanese is as follows;
Though Japanese pharmaceutical companies welcome this decision, no one knows whether the new deadline is enough for them.
3 Likes
The translation of the part of the original document is as follows;
The notification and administrative communication require MAHs to assess the risk of contamination with nitrosamines for the products they manufacture and sell, taking into account known causes of contamination with nitrosamines, to measure the amount of nitrosamines in products with a contamination risk, and to take risk reduction measures, such as setting standards and changing the manufacturing method to reduce the amount of nitrosamines, by October 31, 2024, for products found to contain nitrosamines exceeding the limit.
On the other hand, a new route cause of contamination and generation of nitrosamines has been discovered, which is the reaction between trace amounts of nitrite contained in pharmaceutical excipients and amines derived from active ingredients, etc., making it necessary to re-perform the risk assessment. Furthermore, there are problems with establishing a measurement system for nitrosamines derived from active ingredients (NDSRIs: nitrosamine drug substance-related impurities), including the acquisition of reference standards, and it has become clear that it will be difficult to implement risk reduction measures within the deadline. In light of this situation, we have decided to extend the implementation deadline for risk reduction measures, which was originally scheduled for October 31, 2024, to August 1, 2025.
1 Like
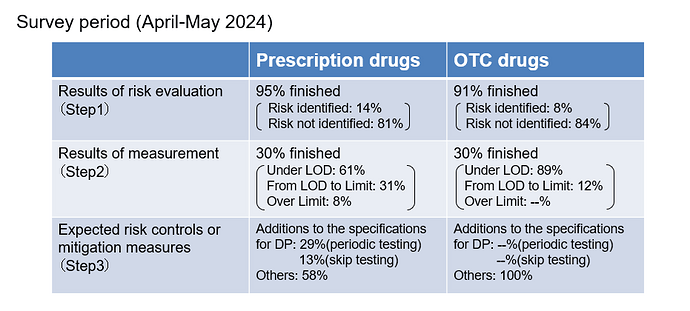
I want to add the latest survey results in Japan.
The link to the original document(written in Japanese) is as follows;
2 Likes