I am posting this question on behalf of a community member:
About APPENDIX C: EXAMPLE OF CONTROL AND SPECIFICATION FOR THE RECOMMENDED ACCEPTABLE INTAKE LIMIT FOR MULTIPLE NITROSAMINES IN ONE DRUG PRODUCT from FDA recent update.
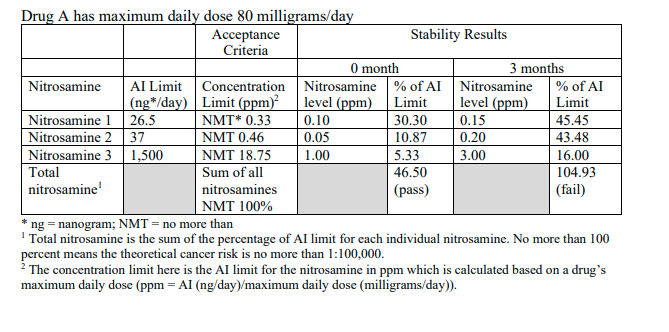
“We have questions regarding the calculations in Table C of the new nitrosamine impurity guidance released by the FDA on September 4, 2024. If a risk assessment has already demonstrated that a particular nitrosamine impurity is of low risk and confirmed that the detected amount of this impurity, both initially and during stability testing, is less than 10% of the AI limit, can it be disregarded in the calculation of the total nitrosamine amount as per the formula in the table below?”
Similar to question above, if we have 2 or 3 nitrosamines in one product, does the 10% principle apply for every nitrosamine or 10% is for the sum of all the nitrosamines?