CDER Nitrosamine Impurity Acceptable Intake Limits update includes acronyms for certain nitrosamines and Table 5 of analytical testing methods. They are minor changes.
NTTP in Sitagliptin was removed from Table 2 and added to Table 1 with the AI of 100ng/day.
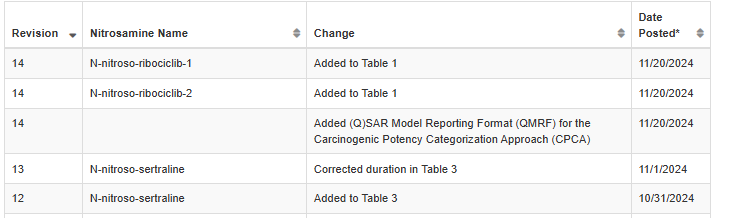
Revision Table
Thank you for sharing @Yosukemino,
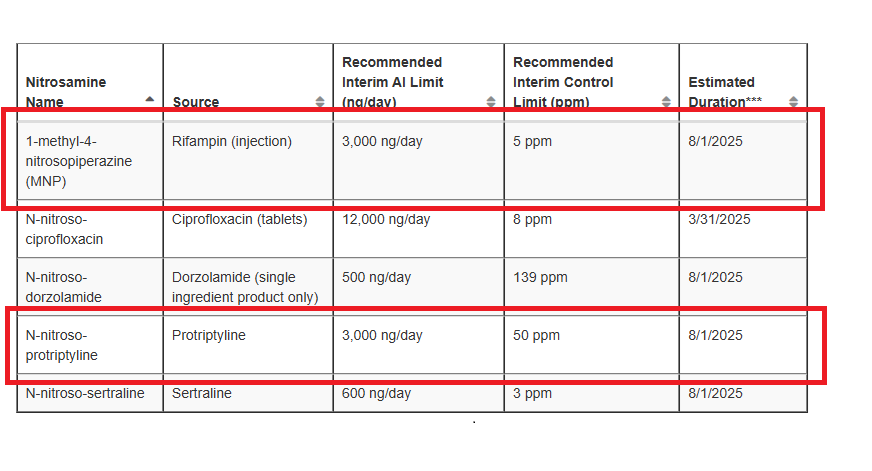
I’m worried about the statement regarding “single ingredient product only.” Does this imply that the limit doesn’t apply to multiple APIs or to multiple detected N-nitroso compounds? I’m unclear on how an additional API without nitrogen could affect the AI of a nitrosamine.
Does anyone have insights on this?
But combination therapies can effectively entail risks for other nitrosamines, e.g. dorzolamide and timolol combination (linked to N-nitroso-timolol). In case multiple NDSRIs would be present in combination products, there is an impact on the lifetime cancer risk evaluation, so the evaluation that was done to support a temporary AI of 500 ng/day cannot automatically be extrapolated without review as both NDSRIs would have to be looked at to calculate the limits.
Thank you for your response Wibon. I agree that multiple N-nitrosamines may impact lifetime cancer risk assessment. However, the FDA restriction specifies “single ingredient product only,” rather than a single NDSRI. From my understanding, this means the 500 ng/day limit wouldn’t apply if there’s more than one API in the product, regardless of whether it can lead to NDSRI formation.
In my case, I do indeed have both Dorzolamide and Timolol in the product. I tested for N-nitroso Timolol, which was below the limit of detection (LOD). Now, I’m wondering if I can apply the 500 ng/day limit for N-nitroso Dorzolamide in this situation. I’d be inclined to say yes, though I am a bit concerned about the FDA’s response.
Dear Grannel,
in cases of more than one nitrosamine in a product you can go with the ‘‘flexible’’ approach where each individual nitrosamine should be calculated as a percentage of its AI limit, such that the sum of all the nitrosamines does not exceed 100 percent.
Appendix C in the below link
https://www.fda.gov/media/141720/download
For the validity of this approach, FDA has a limit of maximum three nitrosamines while EMA has no limitation of the existing nitrosamines number.
best regards
Christos
Pharm Tech published a great review from @ASrinivasan on updated guidance. Thank you, Aloka.
LOL! Yusukemino San, I was hesitating to post it. ![]()
What I have tried to do here is an analysis of what some of the things that the FDA has said translates to for the industry. One thing that came up constantly is a significant workload increase for application holders and DP manuafacturers.
US FDA updated Table 1 on 28/10/2024
Annexure 1_FDA Nitrosamine Impurity Acceptable Intake Limits.pdf (112.7 KB)
: Recommended AI Limits for Certain Hypothetical NDSRIs and Other Identified Nitrosamine Impurities**
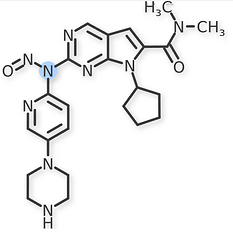
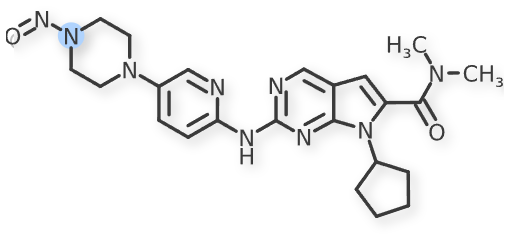
The recent updates added N-nitroso-sertraline to Table 3 with an Interim AI of 600 ng/day. Also, N-nitroso-ribociclib-1 and N-nitroso-ribociclib-2 were added to the Table 1.
(Q)SAR model information for CPCA is available.
N-nitroso-ribociclib-1
N-nitroso-ribociclib-2
- (Q)SAR model reporting format (QMRF) for CPCA, described in Kruhlak NL, et al. Determining recommended acceptable intake limits for N-nitrosamine impurities in pharmaceuticals: Development and application of the Carcinogenic Potency Categorization Approach (CPCA). Regul Toxicol Pharmacol. 2024 Jun; 150:105640.
Dear Yosukemino Nitrosamine Exchange Ambassador,
Greeting!
We are working on n-nitroso Metolazone impurity but solution stability is the issue. We wish to know, is anybody in this forum have idea about in which condition this impurity remains stable at east 12-15 hrs. I would appreciate if get this information.
Thank you
Sanjay Chaudhari
Thank you for asking, @sanjhira. However, I am not sure about the stability of the test solution for n-nitroso Metolazone. To solve this issue, you can make a thread in the category of confirmatory testing. It will help others suffering from the same problem.
I asked Dr. Kruhlak from CDER how to decide the interim limit of nitrosamines. According to her, the value is determined on a case-by-case basis by considering multiple things, such as the amount of nitrosamines in the drug products and the presence or absence of alternatives to avoid drug shortages. This is what I wanted to know!!
N-Nitroso Metolazone is stable upto 12 hrs @ sample cooler temperature (10c) in Water : Methanol (80:20)
Thank you very much for information related to N-nitroso Metolazone solution stability and diluent details
FDA updated Table 3: Recommended Interim AI Limits list. MNP and N-nitroso-protriptyline were added with 3000 ng/day of interim limit, and N-nitroso-duloxetine was removed on 12/31/2024.
Thank you Yosukemino. Is the deletion of duloxetine from the FDA’s interim limit table signifies that the interim limit is no longer applicable, and compliance with the standard limit of 100 ng/day is now required ?