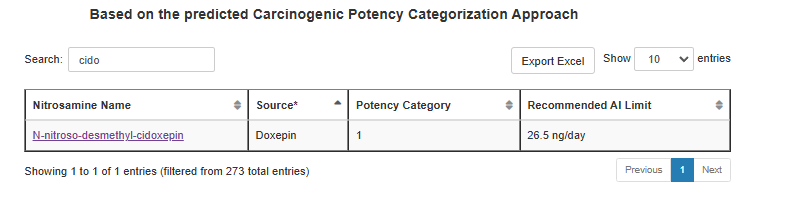
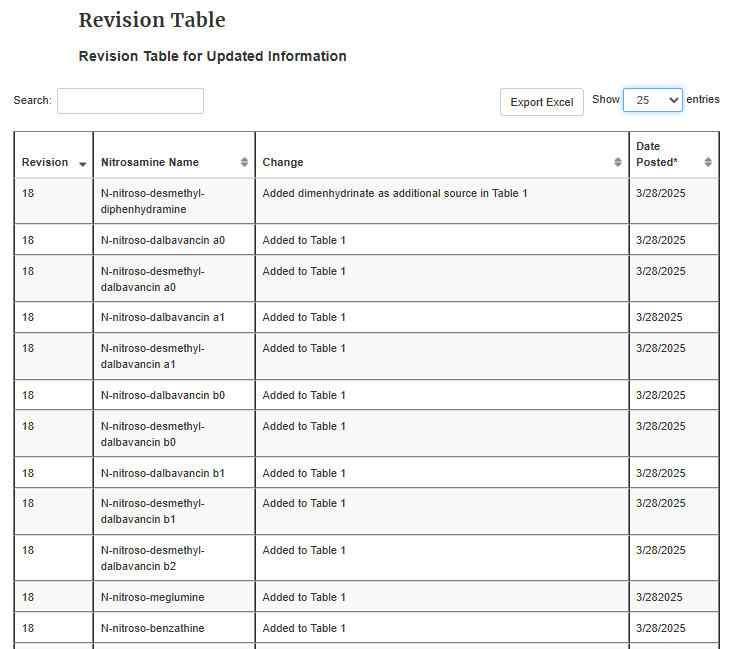
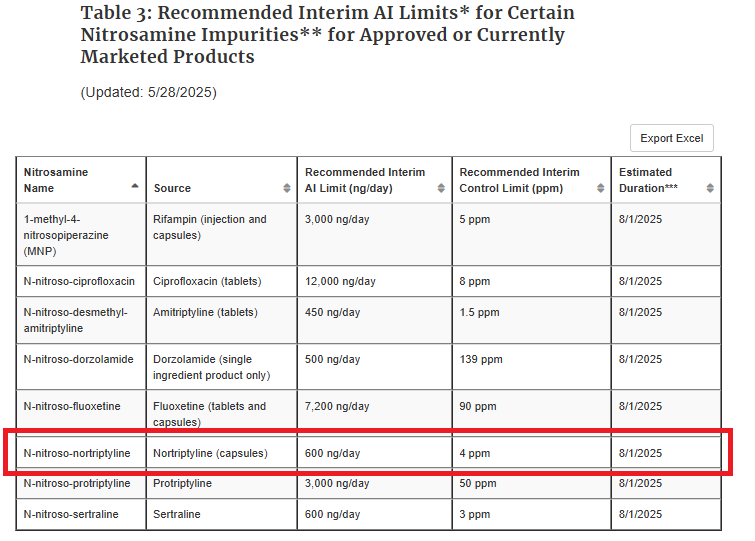
Thank you for asking, @sbasaleh. The interim AI limit of 600 ng/day for N-nitroso-duloxetine expired on 12/31/2024. I added a picture of the previous Table 3, which is now invalid unless there is no additional information.
@Yosukemino - have they also updated table 1? It says that it was updated on the 31st of December - but there isn’t a column for the date when any limit was added/changed.
I couldn’t remember there being a limit for n-nitroso proline previously, as I thought that this was believed to be non carcinogenic, and several others seemed new to me as well.
N-nitroso-proline in Table 1 is not new.
I don’t see new additions, might be only maintenance of the “**” signs to link Table 1 and 3?
Noticed Aripiprazol and Cidoxepin NDSRIs were removed (previous removals due to no marketing API were also not always in the revision history), but can’t say in which version this was.
I agree with Dr. Wybon. I compared the latest version with the 28/10/2024 version that Dr. Gulhane shared, and I found the difference was only N-nitroso-ribociclib-1 and N-nitroso-ribociclib-2.
I guess the revision history is correct. N-nitroso-desmethyl-cidoxepin is in Table 1.
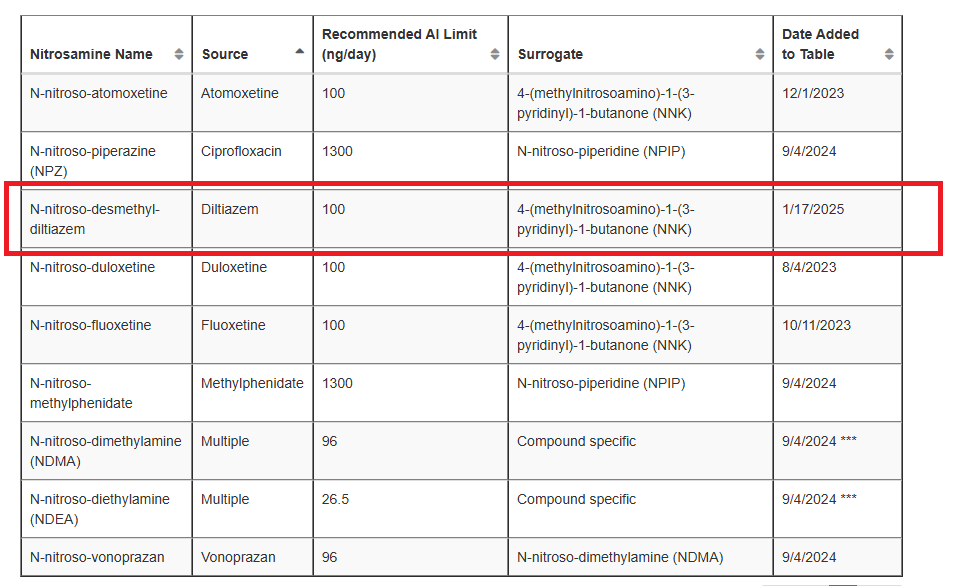
N-nitroso-desmethyl-diltiazem was removed from Table 1 and added to Table 2 with an AI of 100 ng/day.
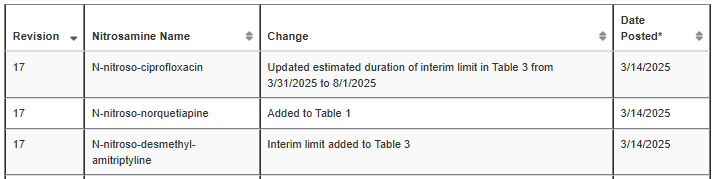
CDER Nitrosamine Impurity Acceptable Intake Limits rev.17
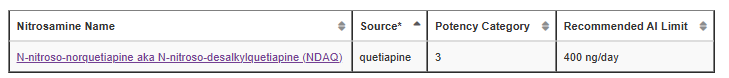
N-nitroso-norquetiapine was added to Table 1 with an AI of 400 ng/day.
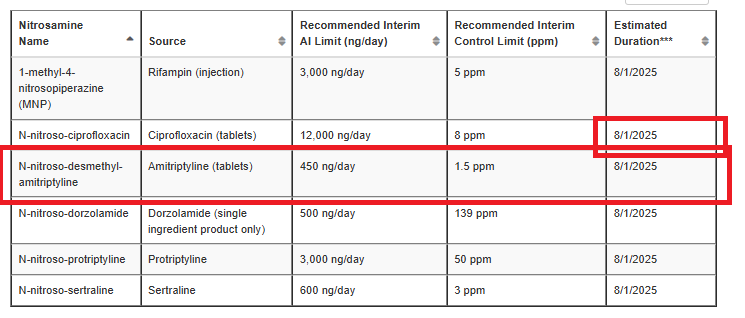
The estimated duration of interim limit for N-nitroso-ciprofloxacin updated in Table 3 from 3/31/2025 to 8/1/2025.
The interim limit for N-nitroso-desmethyl-amitriptyline was added to Table 3.
Thanks Yosuke for the update.
For Amitriptyline, can you please guide on which basis new interim limit proposed by FDA (450ng).
Thank you for asking, @Pooja. I want to share my previous post. I guess the interim limit was determined to avoid drug shortage.
Thanks Yosuke for the information.
Is this interim limit value also depends on toxicological assessment of molecule that any organization submits to FDA authority and then FDA decides the limit.
Now, these nitrosamine impurities are on the AI list. It contains many nitrosamine impurities related to dalbavancin.
N-nitroso-dalbavancin a0
N-nitroso-desmethyl-dalbavancin a0
N-nitroso-dalbavancin a1
N-nitroso-desmethyl-dalbavancin a1
N-nitroso-dalbavancin b0
N-nitroso-desmethyl-dalbavancin b0
N-nitroso-dalbavancin b1
N-nitroso-desmethyl-dalbavancin b1
N-nitroso-desmethyl-dalbavancin b2
N-nitroso-meglumine
N-nitroso-benzathine
Dimenhydrinate is added as the source of N-nitroso-desmethyl-diphenhydramine.
CDER Nitrosamine Impurity Acceptable Intake Limits rev.18
dalbavancin
Hello everyone,
Could anyone please advise on where to obtain reference standards for the nitrosamine impurities associated with Dabavanxin? We manufacture Dabavanxin API and are currently evaluating our testing strategy. Do we need to test for all the related nitrosamine impurities, or is it acceptable—based on reactivity and clearance efficiency analysis—to focus only on those with the highest residual risk?
Any insights or experiences you can share would be greatly appreciated.
Thank you!
Dear daixulin,
personally i would go for testing only for the N-nitroso Dabavanxin (as Dabavanxin is clear a secondary amine) and for NDMA (only because there is a dimethyl amino moiety in Dabavanxin molecule).
If the levels of the N-nitroso Dabavanxin are <10% of the spec. limit then i would not test for any other nitroso-impurity related to it. If the risk for the API nitrosamine is negligible, consequently the risk for the formation of any other related to it nitrosamine impurity is anticipated to be even less. If the results are >10% of the spec. limit, then testing also for the N-desmethyl Dabavanxin is recommended.
best regards
Christos
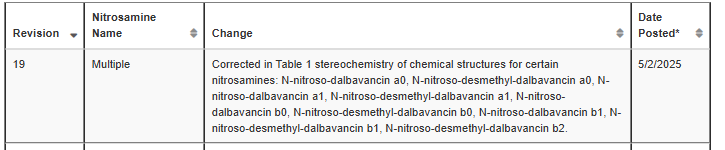
The stereochemistry of chemical structures for certain nitrosamines(dalbavancin impurities) was corrected in Table 1.
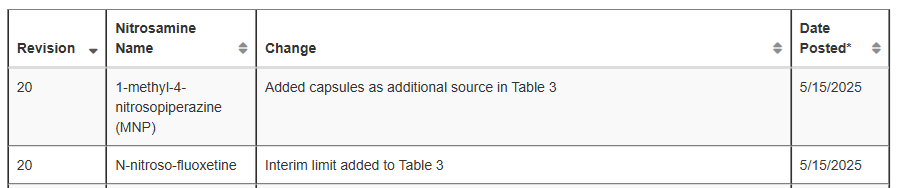
Rifampin capsules has been added as a source of MNP to Table 3.
An interim AI limit of 7200ng/day for N-nitroso-fluoxetine has been added to Table 3.
The Interim Limit of 600ng/day for N-nitroso-nortriptyline has been added.
The AI for N-nitroso-nortriptyline is 26.5ng/day.
Hello everyone, could someone explain why is there a diffference between N-nitroso-desmethyl-amitriptyline and N-nitroso-nortriptiline?
As I see they are the same nitrosamine? Right?
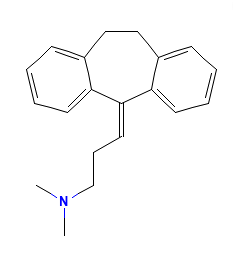
this is the structure of Amitriptyline
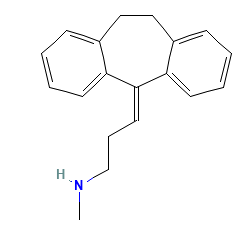
This is the structure of Nortriptyline, or Amitriptyline EP impurity C
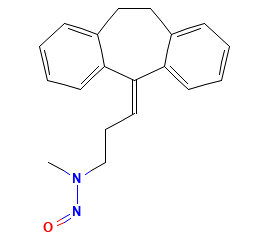
That would be the nitrosamine in question. Right?
Why FDA is proposing two different acceptable intakes for the same nitrosamine?
Nortriptyline (= desmethyl-amitryptyline) and amitriptyline are both tricyclic antidepressants.
They have indeed one shared nitrosamine, N-nitroso-nortriptyline = N-nitroso-desmethyl-amitryptyline (nitroso-API in one case and nitroso-desmethyl API in the other case).
The nuance is not about there being different nitrosamines or different “final” limits, but about the temporary exception table 3 of FDA being not only nitrosamine-specific, but also product-specific (as it is designed based on shortage prevention).
In this case a higher temporary limit is in place for nortriptyline capsules but not for amitriptyline products via Table 3.
See also footnote of table 3:
With respect to FDA-recommended interim control limits, FDA generally does not intend to object to the distribution by manufacturers and applicants of drug products that contain nitrosamine levels at or below the recommended interim control limit, until the date identified in the “Estimated Duration” column. However, if FDA becomes aware that the shortage risk has been alleviated, FDA may provide additional notification or may reconsider its recommended interim control limit.
So, apparently there is a risk for impact on patient access to nortriptyline capsules that the temporary limit helps to relief.
For amitriptyline products there might not be such shortage risk without a higher limit being generally in place via the table 3.
This would not be surprising as amitriptyline is the tertiary amine API and not the secondary.
Hi C. Wybon,
Thanks for your reply—it was really helpful and aligned quite well with my previous thoughts. That said…
I understand there’s a shortage in the market for some products, and that in certain cases, the chemical structure and formulation can lead to increased nitrosamine formation, which may be impossible to mitigate during the manufacturing process.
However, the decision still seems somewhat arbitrary and unclear. There’s no explanation as to why a nitrosamine would be considered less potent simply because it’s in a capsule rather than a tablet.
Instead of recommending two different limits for the same nitrosamine, it would make more sense to establish a single exposure limit. My point is: just because I can’t reduce nitrosamine formation in my product doesn’t mean the nitrosamine itself is any less potent. So, if a limit of 600 is considered safe, it should apply to any product containing that nitrosamine—regardless of its origin or formulation
I’ve an amitriptyline drug in capsule (not tablet), so do you reckon I should use 600ng/day?
Thanks again for your time,
regards