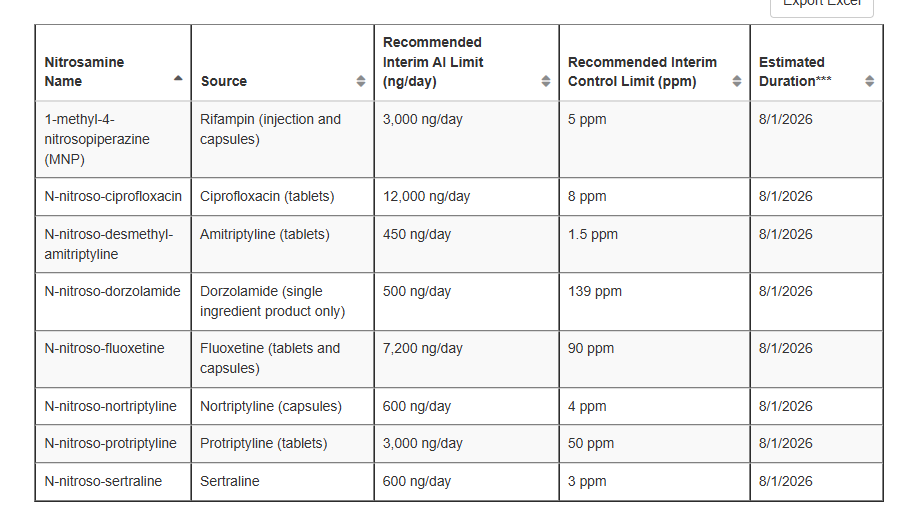
I don’t think Table 3 is designed at this stage to reflect the real or confirmed potency, otherwise there would be no reason to have it as a temporary table or product-specific.
There are certain regards included in Table 3 values that have nothing to do with the “real” daily lifetime AI value, Table 3 is the result of a balancing exercise.
The single exposure limit for the nitrosamine remains CPCA category 1, until any other final limit is published, these are just temporary actions to prevent shortages as the footnote indicates. They give some hints maybe about temporary (!) limit design considerations, but not all, part of the value is product-specific shortage prevention.
It’s not excluded that requests to widen the scope of Table 3 are successful. December 2024, MNP was for injections in Table 3, in May 2025 capsules were added as additional source.
You have to ask your self if you would make a case for 450 ng/day (amitriptyline tablets entry) or 600 ng/day (nortriptyline capsules) or another value. I guess this starts with the question what limit you need practically so that your product is not in shortage, and for what limit would an optimal risk/benefit balance be arguable based on best available science?
If a lower limit would be doable, you might consider if you have perhaps data to build a case for a Table 2 entry instead, as this is more permanent.