@Nitrosamines_Analyzer @trust_level_2 @trust_level_3 @trust_level_4
Happy Monday Everyone!
On April 9–10, the FDA hosted its Generic Drugs Forum, a cornerstone event that goes far beyond regulatory updates — it reflects the agency’s current thinking, concerns, and future direction for generics. Unsurprisingly, Nitrosamine Impurities featured prominently on the agenda.
But here’s the part we really need to unpack as a community.
![]() Our founding member, @Yosukemino, has generously shared first-hand insights, the full agenda, and workshop recording here:
Our founding member, @Yosukemino, has generously shared first-hand insights, the full agenda, and workshop recording here:
![]() https://nitrosamines.usp.org/t/generic-drugs-forum-gdf-2025/12789?u=naiffer_host
https://nitrosamines.usp.org/t/generic-drugs-forum-gdf-2025/12789?u=naiffer_host
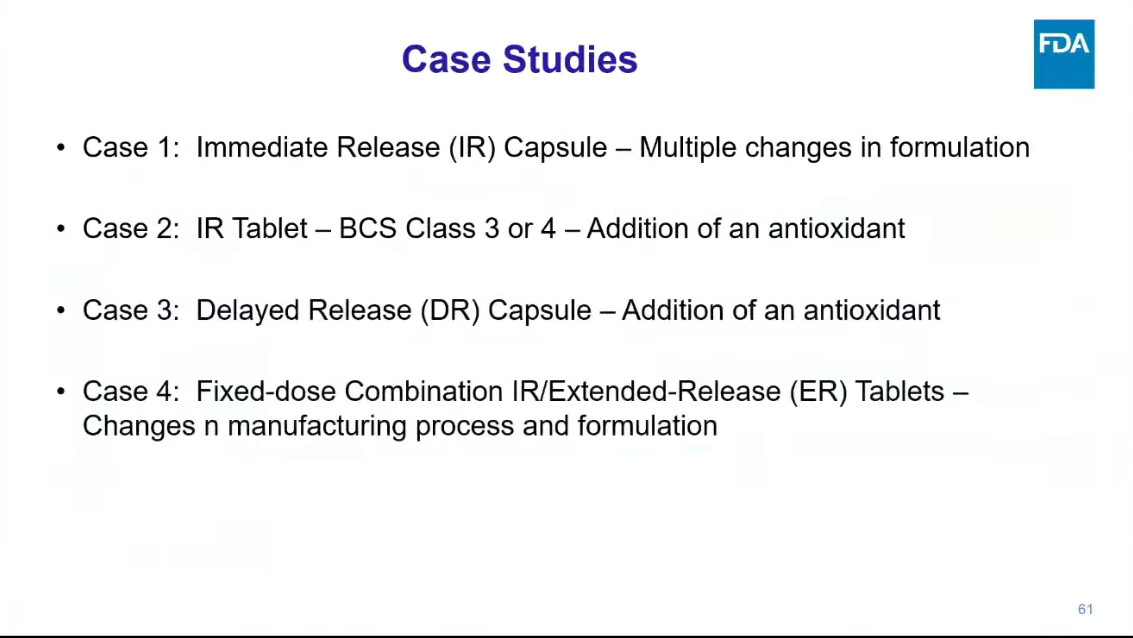
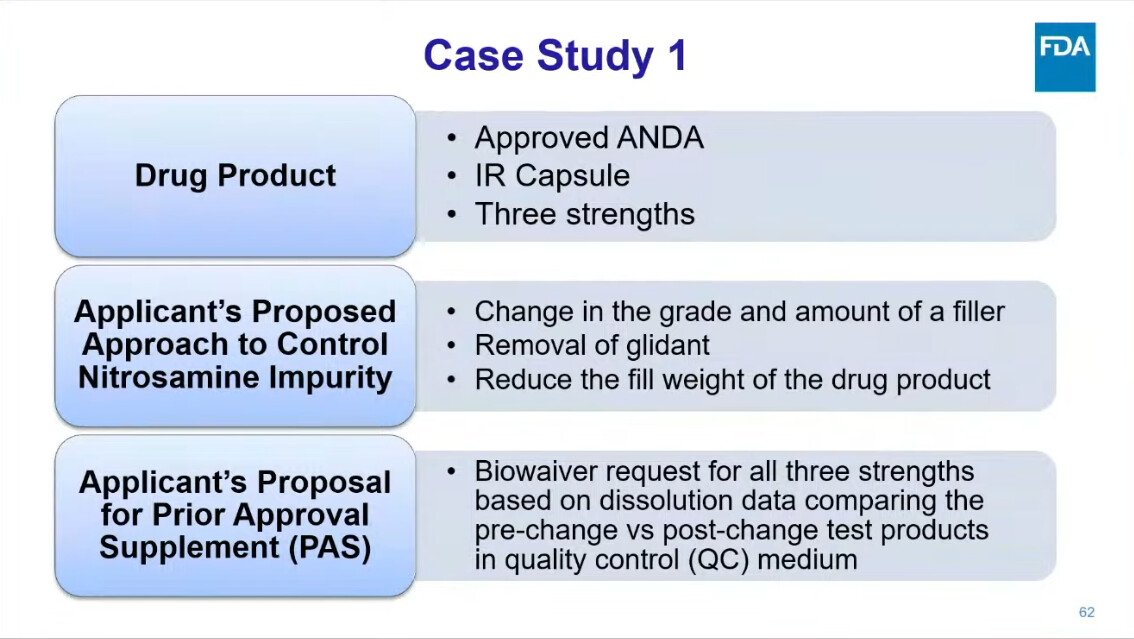
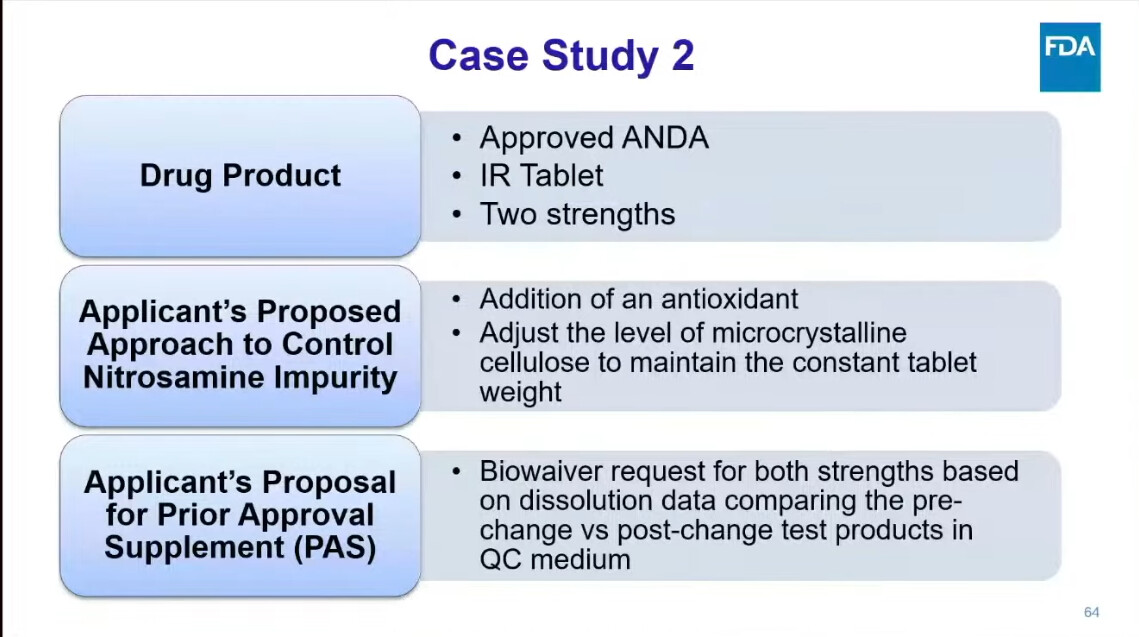
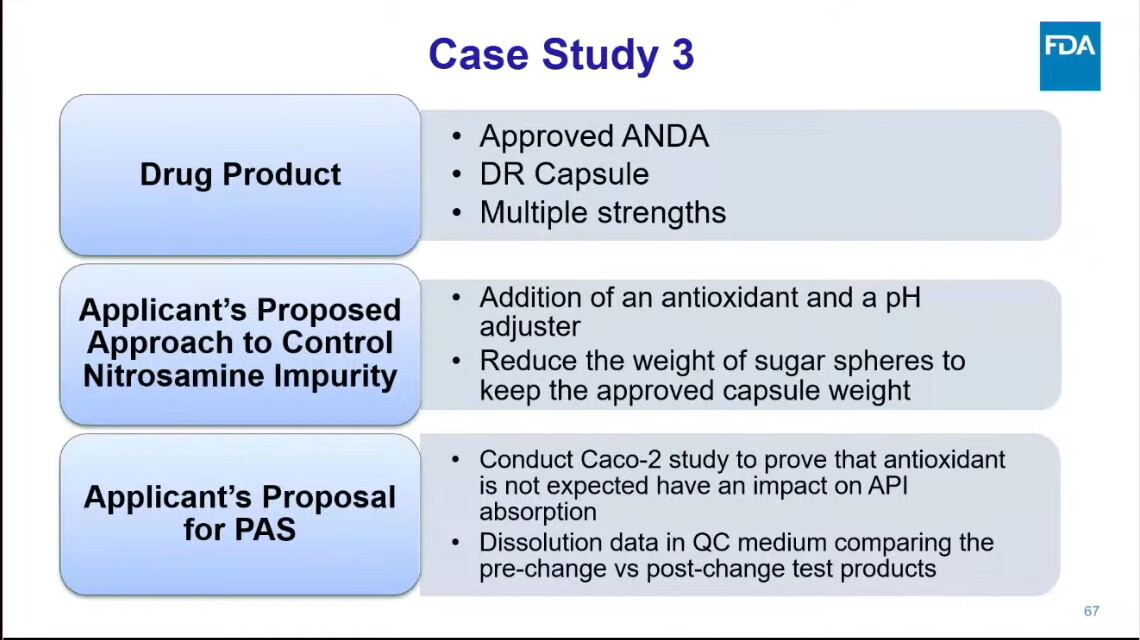
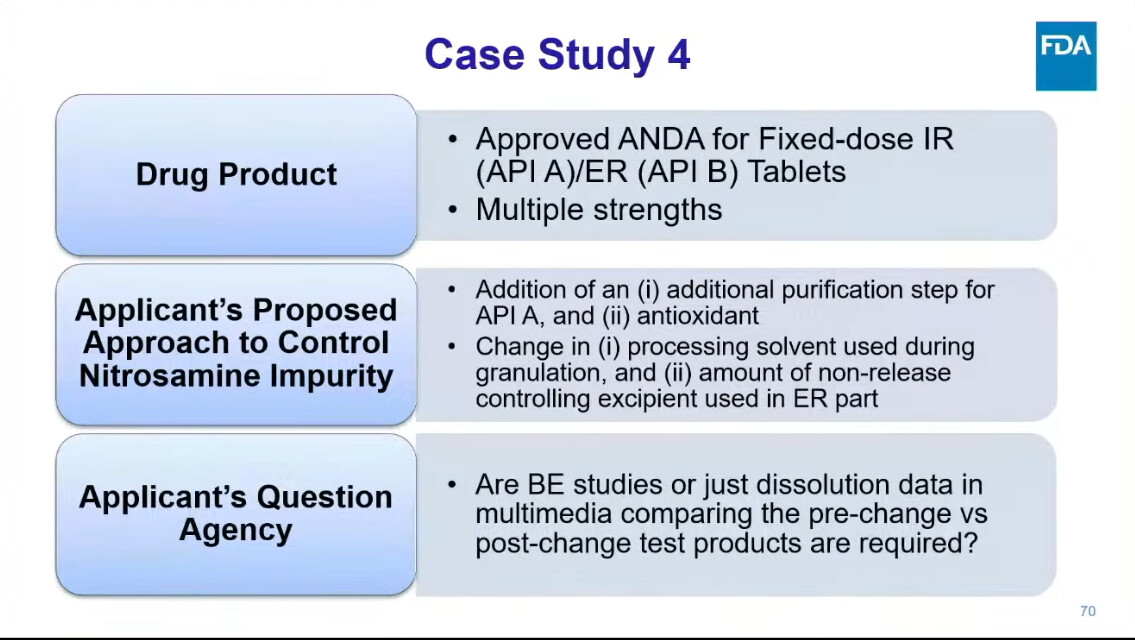
One presentation, in particular, deserves our undivided attention: Dr. Kaur from FDA’s Division of Bioequivalence walked through real case studies from drug applications — showing how sponsors tried to mitigate nitrosamines, and what FDA actually recommended in response.
![]() Why is this important?
Why is this important?
Because these are not theoretical exercises — they reflect the actual regulatory mindset shaping the future of nitrosamines strategies.
So here’s where I turn to you, our community:
- Were these recommendations aligned with your expectations based on past interactions with FDA?
- Did you notice any shift in tone or regulatory stringency?
- Are we, as an industry, truly aligned with what FDA expects in terms of nitrosamine mitigation and bioequivalence?
Let’s start a conversation. The answers are in the details — and they might just reshape how the community prepare for the next filing.
![]() Drop your thoughts below or join the discussion in the link above. Your insight could unlock new perspectives for all of us.
Drop your thoughts below or join the discussion in the link above. Your insight could unlock new perspectives for all of us.