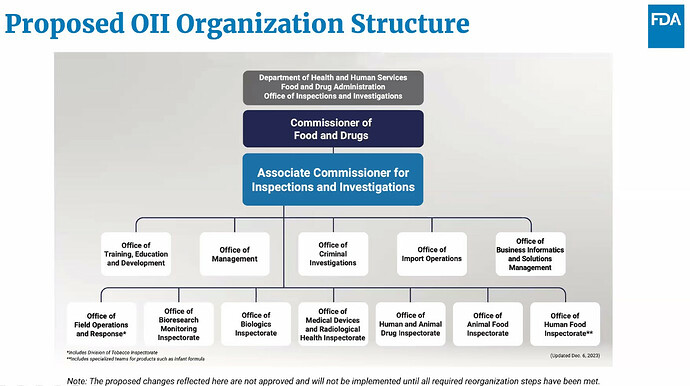
Top US Food and Drug Administration (FDA) officials have outlined the agency’s plans to reform its human foods program and transform its Office of Regulatory Affairs (ORA) into the Office of Inspections and Investigations (OII). They noted that about 1,500 ORA staff would be reassigned to product centers to work directly on inspections and investigations as part of the proposed reorganization
Webinar and Slides:
Source: https://www.raps.org/News-and-Articles/News-Articles/2024/1/FDA-leaders-detail-reorg-plans